Abstract
Aptamer-modified SiC quantum dots (DNA-SiC QDs) as fluorescent aptasensor are described for the determination of Proteus mirabilis. The SiC QDs were synthesized through one-pot hydrothermal method with particle sizes of about 14 nm. The amino-modified aptamers against P. mirabilis were conjugated to the surfaces of SiC QDs for bacteria recognition. The aptamer with an affinity for target protein can bound to P. mirabilis and this causes a decrease in the fluorescence intensity of DNA-SiC QDs. P. mirabilis levels were tested by the aptasensor within 35 min with fluorescence excitation/emission maxima at 320/420 nm. The linear range is from 103 to 108 CFU mL−1 and the limit of detection is 526 CFU mL−1 (S/N = 3). The aptasensor was used for determination of P. mirabilis in pure milk samples and obtained good accuracy (87.6–104.5%) and recovery rates (85–110.2%) were obtained. The detection in simulated forensic identification samples (pure milk, milk powder, blood, and urine) obtained gave satisfactory coincidence rates with the method of bacterial isolation and identification as standard. These results demonstrate that the fluorescent aptasensor is a potential tool for identification of P. mirabilis in forensic food poisoning cases.

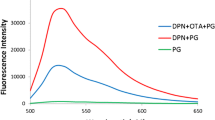
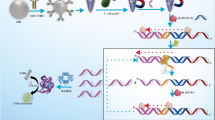
Determination of P. mirabilis is based on SiC QDs fluorescence aptasensor. The SiC QDs with plentiful carboxyl groups on the surface can be synthesized via one-pot hydrothermal route. After activated by EDC/NHS, the SiC QDs can bind to aptamer to form fluorescence aptasensors. When the target P. mirabilis exists, the fluorescence of aptasensor will be quenched and the determination of the P. mirabilis based on the fluorescence change can be analyzed.





Similar content being viewed by others
References
Marien T, Miller NL (2015) Treatment of the infected stone. Urol Clin North Am 42(4):459–472. https://doi.org/10.1016/j.ucl.2015.05.009
Norsworthy AN, Pearson MM (2017) From catheter to kidney stone: the uropathogenic lifestyle of Proteus mirabilis. Trends Microbiol 25(4):304–315. https://doi.org/10.1016/j.tim.2016.11.015
Madana J, Yolmo D, Kalaiarasi R, Gopalakrishnan S, Sujatha S (2011) Microbiological profile with antibiotic sensitivity pattern of cholesteatomatous chronic suppurative otitis media among children. Int J Pediatr Otorhinolaryngol 75(9):1104–1108. https://doi.org/10.1016/j.ijporl.2011.05.025
Spagnolo EV, Mondello C, Roccuzzo S et al (2019) A unique fatal case of Waterhouse-Friderichsen syndrome caused by Proteus mirabilis in an immunocompetent subject: case report and literature analysis. Medicine (Baltimore) 98(34):e16664. https://doi.org/10.1097/MD.0000000000016664
Cooper KE, Davies J, Wiseman J (2005) An investigation of an outbreak of food poisoning associated with organisms of the Proteus group. J Pathol Bacteriol 52(1):91–98. https://doi.org/10.1002/path.1700520107
Wang Y, Zhang S, Yu J, Zhang H, Yuan Z, Sun Y, Zhang L, Zhu Y, Song H (2010) An outbreak of Proteus mirabilis food poisoning associated with eating stewed pork balls in brown sauce, Beijing. Food Control 21(3):302–305. https://doi.org/10.1016/j.foodcont.2009.06.009
Keisam S, Tuikhar N, Ahmed G, Jeyaram K (2019) Toxigenic and pathogenic potential of enteric bacterial pathogens prevalent in the traditional fermented foods marketed in the northeast region of India. Int J Food Microbiol 296:21–30. https://doi.org/10.1016/j.ijfoodmicro.2019.02.012
Blackburn CW, McCarthy JD (2000) Modifications to methods for the enumeration and detection of injured Escherichia coli O157:H7 in foods. Int J Food Microbiol 55(1–3):285–290. https://doi.org/10.1016/s0168-1605(00)00205-1
Syed MA, Jamil B (2018) Aptamers and aptasensors as novel approach for microbial detection and identification: an appraisal. Curr Drug Targets 19(13):1560–1572. https://doi.org/10.2174/1389450119666180105115429
Yi J, Wu P, Li G, Xiao W, Li L, He Y, He Y, Ding P, Chen C (2019) A composite prepared from carboxymethyl chitosan and aptamer-modified gold nanoparticles for the colorimetric determination of Salmonella typhimurium. Mikrochim Acta 186(11):711. https://doi.org/10.1007/s00604-019-3827-5
Zhang H, Ma X, Liu Y, Duan N, Wu S, Wang Z, Xu B (2015) Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens Bioelectron 74:872–877. https://doi.org/10.1016/j.bios.2015.07.033
Wu WH, Li M, Wang Y, Ouyang H-x, Wang L, Li C-x, Cao Y-c, Meng Q-h, Lu J-x (2012) Aptasensors for rapid detection of Escherichia coliO157:H7 and Salmonella typhimurium. Nanoscale Res Lett 7(1):658. https://doi.org/10.1186/1556-276X-7-658
Cao Y, Dong H, Pu S, Zhang X (2018) Photoluminescent two-dimensional SiC quantum dots for cellular imaging and transport. Nano Res 11(8):4074–4081. https://doi.org/10.1007/s12274-018-1990-3
Savory N, Lednor D, Tsukakoshi K, Abe K, Yoshida W, Ferri S, Jones BV, Ikebukuro K (2013) In silico maturation of binding-specificity of DNA aptamers against Proteus mirabilis. Biotechnol Bioeng 110(10):2573–2580. https://doi.org/10.1002/bit.24922
Korolev N, Vorontsova OV, Nordenskiöld L (2007) Physicochemical analysis of electrostatic foundation for DNA-protein interactions in chromatin transformations. Prog Biophys Mol Biol 95(1–3):23–49. https://doi.org/10.1016/j.pbiomolbio.2006.11.003
Zakharko Y, Botsoa J, Alekseev S, Lysenko V, Bluet JM, Marty O, Skryshevsky VA, Guillot G (2010) Influence of the interfacial chemical environment on the luminescence of 3C[single bond]SiC nanoparticles. J Appl Phys 107(1):013503. https://doi.org/10.1063/1.3273498
Wu X, Zhang Z, Li J, You H, Li Y, Chen L (2015) Molecularly imprinted polymers-coated gold nanoclusters for fluorescent detection of bisphenol A. Sensors Actuators B Chem 211:507–514. https://doi.org/10.1016/j.snb.2015.01.115
Petoral RM, Yazdi GR, Vahlberg C et al (2007) Surface functionalization of SiC for biosensor applications. Mater Sci Forum 556-557:957–960. https://doi.org/10.4028/www.scientific.net/MSF.556-557.957
Li X, Wang X, Bondokov R, Morris J, An YH, Sudarshan TS (2005) Micro/nanoscale mechanical and tribological characterization of SiC for orthopedic applications. J Biomed Mater Res B Appl Biomater 72(2):353–361. https://doi.org/10.1002/jbm.b.30168
Shei SC, Chiang WJ, Chang SJ (2015) Synthesis of CuInS2 quantum dots using polyetheramine as solvent. Nanoscale Res Lett 10:122. https://doi.org/10.1186/s11671-015-0789-3
Pan D, Zhang J, Li Z, Wu M (2010) Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater 22(6):734–738. https://doi.org/10.1002/adma.200902825
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22(46):24230. https://doi.org/10.1039/C2JM34690G
Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, Jia Q, Niu G, Huang X, Zhou H, Meng X, Wang P, Lee CS, Zhang W, Han X (2014) A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat Commun 5:4596. https://doi.org/10.1038/ncomms5596
Zhang L, Chen C, Li W, Gao G, Gong P, Cai L (2016) Living cell multilifetime encoding based on lifetime-tunable lattice-strained quantum dots. ACS Appl Mater Interfaces 8(21):13187–13191. https://doi.org/10.1021/acsami.6b03795
Dai W, Dong H, Fugetsu B, Cao Y, Lu H, Ma X, Zhang X (2015) Tunable fabrication of molybdenum disulfide quantum dots for intracellular MicroRNA detection and multiphoton bioimaging. Small 11(33):4158–4164. https://doi.org/10.1002/smll.201500208
Li X, Ding Y, Ling J, Yao W, Zha L, Li N, Chang Y, Wang Y, Cai J (2019) Bacteria-targeting BSA-stabilized SiC nanoparticles as a fluorescent nanoprobe for forensic identification of saliva. Mikrochim Acta 186(12):756. https://doi.org/10.1007/s00604-019-3890-y
Liu Y, Cao Y, Wang T, Dong Q, Li J, Niu C (2019) Detection of 12 common food-borne bacterial pathogens by TaqMan real-time PCR using a single set of reaction conditions. Front Microbiol 10:222. https://doi.org/10.3389/fmicb.2019.00222
Chen J, Xu Y, Yan H, Zhu Y, Wang L, Zhang Y, Lu Y, Xing W (2018) Sensitive and rapid detection of pathogenic bacteria from urine samples using multiplex recombinase polymerase amplification. Lab Chip 18(16):2441–2452. https://doi.org/10.1039/c8lc00399h
Yang D, Zhou H, Dina NE, Haisch C (2018) Portable bacteria-capturing chip for direct surface-enhanced Raman scattering identification of urinary tract infection pathogens. R Soc Open Sci 5(9):180955. https://doi.org/10.1098/rsos.180955
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81772025 and 81772026).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 337 kb)
Rights and permissions
About this article
Cite this article
Yao, W., Shi, J., Ling, J. et al. SiC-functionalized fluorescent aptasensor for determination of Proteus mirabilis. Microchim Acta 187, 406 (2020). https://doi.org/10.1007/s00604-020-04378-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04378-5