Abstract
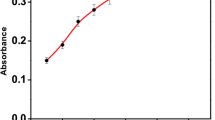
We report on a simple, rapid and sensitive colorimetric assay for the quantitation of cationic surfactants (CS+) in domestic effluent, municipal waste and surface water samples. The method is based on the aggregation of tartrate-capped gold nanoparticles (AuNPs) through both electrostatic and hydrophobic interactions that occur between CS+ and AuNPs. Aggregation results in a color change from pink to blue which is due to a shift in the localized surface plasmon resonance band. The detergent cetylpyridinium chloride (CPC) was chosen as a model compound for optimization of the method for determination of a CS+. Under optimized experimental conditions (pH 9.0; reaction time 5 min; 25 nM concentration of AuNPs), a linear calibration plot was obtained for quantitative determination of CPC, cetyltrimethylammonium bromide (CTAB), dodecyltrimethyl ammonium bromide (DTAB) in the range of 10–500, 10–200 and 10–300 ngmL−1, respectively and the limit of detection was 3 ngmL−1 for CPC, CTAB and DTAB.

The method is based on the aggregation of tartrate-capped AuNPs through both electrostatic and hydrophobic interactions that occur between CS+ and AuNPs. Aggregation results in a color chenge from pink to blue.






Similar content being viewed by others
References
Tadros TF (2005) Applied surfactants: principle and applications. Wiley-VCH, Wieinheim
Patel KS, Hoffmann P (2004) Determination of cationic surfactants in environmental samples by flow injection analysis. Microchim Acta 147:273–278
Merino F, Rubio S, Perez-Bendito D (2003) Mixed aggregate-based acid-induced cloud-point extraction and ion-trap liquid chromatography–mass spectrometry for the determination of cationic surfactants in sewage sludge. J Chromatogr A 998:143–154
Norberg J, Thordarson E, Mathiasson L, Jonsson JA (2000) Microporous membrane liquid–liquid extraction coupled on-line with normal-phase liquid chromatography for the determination of cationic surfactants in river and waste water. J Chromatogr A 869:523–529
Drobeck HP (1994) In: Singer EJ, Cross J (eds) Cationic surfactants, analytical and biological evaluation. Marcel Dekker, New York, p 61
Kamaya M, Kaneko Y, Nagashima K (1999) Simple method for spectrophotometric determination of cationic surfactants without liquid–liquid extraction. Anal Chim Acta 384:215–218
Li S, Zhao S (2004) Spectrophotometric determination of cationic surfactants with benzothiaxolyldiazoaminoazobenzene. Anal Chim Acta 501:99–102
Patel R, Patel KS (1999) Simple and specific method for flow injection analysis determination of cationic surfactants in environmental and commodity samples. Talanta 48:923–931
Wulf V, Wienand N, Wirtz M, Kling HW, Gab S, Schmitz OJ (2010) Analysis of special surfactants by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. J Chromatogr A 1217:749–754
Hind AR, Bhargava SK, Grocott SC (1997) Quantitation of alkyltrimethylammonium bromides in bayer process liquors by gas chromatography and gas chromatography–mass spectrometry. J Chromatogr A 765:287–293
Ding WH, Tsai PC (2003) Determination of alkyltrimethylammonium chlorides in river water by gas chromatography/ion trap mass spectrometry with electron impact and chemical ionization. Anal Chem 75:1792–1797
Zhao Q, Simmons J, Conte ED (2006) Investigation of a variety of cationic surfactants attached to cation-exchange silica for hydrophobicity optimization in admicellar solid-phase extraction for high-performance liquid and gas chromatography. J Chromatogr A 1132:1–7
Peng XT, Shi ZG, Feng YQ (2011) Rapid and high-throughput determination of cationic surfactants in environmental water samples by automated on-line polymer monolith microextraction coupled to high performance liquid chromatography–mass spectrometry. J Chromatogr A 1218:3588–3594
Shrivas K, Wu HF (2007) A rapid, sensitive and effective quantitative method for simultaneous determination of cationic surfactant mixtures from river and municipal wastewater by direct combination of single-drop microextraction with AP-MALDI mass spectrometry. J Mass Spectrom 42:1637–1644
Shrivas K, Wu HF (2008) Oxidized multiwalled carbon nanotubes for quantitative determination of cationic surfactants in water samples using atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry. Anal Chim Acta 628:198–203
Oztekin N, Erim FB (2005) Determination of cationic surfactants as the preservatives in an oral solution and a cosmetic product by capillary electrophoresis. J Pharm Biomed Anal 37:1121–1124
Heinig K, Vogt C, Werner G (1997) Determination of cationic surfactants by capillary electrophoresis with indirect photometric detection. J Chromatogr A 781:17–22
Hui-Feng S, Hase T, Hata N, Kasahara I, Taguchi S (2011) Membrane filters as solid-phase extraction media for the spectrophotometric determination of cationic surfactants in river water and sediment. Anal Sci 17:i197–i200
Motl NE, Smith AF, DeSantisa CJ, Skrabalak SE (2014) Engineering plasmonic metal colloids through composition and structural design. Chem Soc Rev 43:3823–3834
Zhang JS, Noguez C (2008) Plasmonic optical properties and applications of metal nanostructures. Plasmonics 3:127–150
Chen GH, Chen WY, Yen YC, Wang CW, Chang HT, Chen CF (2014) Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal Chem 86:6843–6849
Xu X, Daniel WL, Wei W, Mirkin CA (2010) Colorimetric Cu2+ detection using DNA-modified gold-nanoparticle aggregates as probes and click chemistry. Small 6:623–626
Liu Y, Liu Y, Li Z, Liu J, Xu L, Liu X (2015) An unusual red-to-brown colorimetric sensing method for ultrasensitive silver(I) ion detection based on a non-aggregation of hyperbranched polyethylenimine derivative stabilized gold nanoparticles. Analyst 140:5335–5343
Thanh TK, Rosenzweig Z (2002) Development of an aggregation-based immunoassay for anti-protein a using gold nanoparticles. Anal Chem 74:1624–1628
Ghasemi F, Hormozi-Nezhad MR, Mahmoudi M (2015) A colorimetric sensor array for detection and discrimination of biothiols based on aggregation of gold nanoparticles. Anal Chim Acta 882:58–67
Jiang Y, Zhao H, Lin Y, Zhu N, Ma Y, Mao L (2010) Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew Chem Int Ed 49:4800–4804
Deng D, Xia N, Li S, Xu C, Sun T, Pang H, Liu L (2012) Simple, fast and selective detection of adenosine triphosphate at physiological pH using unmodified gold nanoparticles as colorimetric probes and metal ions as cross-linkers. Probes 12:15078–15087
Menon SK, Mistry BR, Joshi KV, Sutariya PG, Patel RV (2012) Analytical detection and method development of anticancer drug gemcitabine HCl using gold nanoparticles. Spectrochim Acta A 94:235–242
Storhoff JJ, Lazarides AA, Mirkin CA, Letsinger RL, Mucic RC, Schatz GC (2000) What controls the optical properties of DNA-linked gold nanoparticle assemblies? J Am Chem Soc 122:4640–4650
Wang XX, Liu JM, Jiang SL, Jiao L, Lin LP, Cui ML, Zhang XY, Zhang LH, Zheng ZY (2013) Non-aggregation colorimetric probe for detecting vitamin C based on surface plasmon resonance of gold nanorods. Sensors Actuators B 182:205–210
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of Au colloid monolayers. Anal Chem 67:735–743
Keith LH (1991) Environmental sampling and guide. Lewis Publishers, Boca Raton
Kelly K, Coronado E, Zhao L, Schatz G (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677
Daniel M, Astruc A (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Desireddy A, Conn BE, Guo J, Yoon B, Barnett RN, Monahan BM, Kirschbaum K, Griffith WP, Whetten RL, Landman U, Bigioni TP (2013) Ultrastable silver nanoparticles. Nature 501:399–402
Lan Q, Liu C, Yang F, Liu S, Xu J, Sun D (2007) Synthesis of bilayer oleic acid-coated Fe3O4 nanoparticles and their application in pH-responsive pickering emulsions. J Colloid Interface Sci 310:260–269
Max JJ, Chapados C (2004) Infrared spectroscopy of aqueous carboxylic acids: comparison between different acids and their salts. J Phys Chem A 108:3324–3337
Agrawal K, Agnihotri G, Shrivas K, Mundhara GL, Patel KS, Hoffmann P (2004) Flow injection analysis determination of cationic surfactants in environmental samples. Microchim Acta 147:273–278
Kostic DA, Mitic SS, Naskovic DC, Zarubica AR, Mitic MN (2012) Determination of benzalkonium chloride in nasal drops by high-performance liquid chromatography. E-J Chem 9:1599–1604
Wang J, Lu J, Zhang L, Hu Y (2003) Determination of cetylpyridinium chloride and tetracaine hydrochloride in buccal tablets by RP-HPLC. J Pharm Biomed Anal 32:381–386
Kuong CL, Chen WY, Chen YC (2007) Semi-quantitative determination of cationic surfactants in aqueous solutions using gold nanoparticles as reporter probes. Anal Bioanal Chem 387:2091–2099
Acknowledgments
We would like to thank the Department of Science Technology, New Delhi for awarding Kamlesh Shrivas a fast track project (NO.SB/FT/CS-128/2012) to furnish this research work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 205 kb)
Rights and permissions
About this article
Cite this article
Shrivas, K., Sahu, S., Ghorai, A. et al. Gold nanoparticles-based colorimetric determination of cationic surfactants in environmental water samples via both electrostatic and hydrophobic interactions. Microchim Acta 183, 827–836 (2016). https://doi.org/10.1007/s00604-015-1689-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1689-z