Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) and metabolic dysfunction associated fatty liver disease (MAFLD) have important associations with cardiovascular disease (CVD). The main objective of this study was to compare the frequency of incidence rate of CVD in the NAFLD or MAFLD patients utilizing a large claims database.
Methods
Using the JMDC database from April 2013 to March 2019, we retrospectively analyzed data for 1,542,688 and 2,452,949 people to estimate the relationship between CVD and NAFLD, MAFLD, respectively.
Results
The incidence rates of CVD were 0.97 (95% CI 0.94–1.01) and 2.82 (95% CI 2.64–3.01) per 1000 person-years in the non-NAFLD and NAFLD groups, respectively, and 1.01 (95% CI 0.98–1.03) and 2.69 (95% CI 2.55–2.83) per 1000 person-years in the non-MAFLD and MAFLD groups, respectively. The overall prevalence of hypertriglyceridemia and diabetes mellitus (DM) was 13.1, and 4.2%, respectively, in the non-NAFLD group and 63.6, and 20.2%, respectively, in the NAFLD group. The overall prevalenceof hypertriglyceridemia and DM was 13.6 and 4.3%, respectively, in the non-MAFLD group and 64.1, and 20.6%, respectively, in the MAFLD group. HRs for CVD increased with hypertriglyceridemia and DM.
Conclusions
Results indicated that incident rate of CVD increased with NAFLD/MAFLD; the complication rate of DM and hypertriglyceridemia among NAFLD/MAFLD patients is high and may affect the development of CVD.
Similar content being viewed by others
Introduction
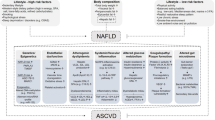
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide with a global prevalence of 25.2% and a prevalence of 29.6% in Asia [1, 2]. NAFLD is regarded as a hepatic component of metabolic syndrome and is associated with other risk factors for metabolic syndrome, such as obesity, diabetes mellitus (DM), and dyslipidemia [1, 3]. Recently, fatty liver caused by nutritional metabolic disorders regardless of other chronic liver diseases has been proposed as a new liver disease concept, “metabolic dysfunction associated fatty liver disease (MAFLD)" [4]. Cardiovascular disease (CVD) has been reported as the most important cause of death, followed by non-liver malignancy and complications of cirrhosis (along with hepatocellular carcinoma and liver transplantation) in NAFLD patients [5, 6]. Furthermore, the accumulation of fat in the liver is reported to be independently associated with coronary plaques, especially non-calcified plaques [7], and both hepatic steatosis and fibrosis are significantly associated with diastolic heart dysfunction [8].
Multiple reports have indicated that NAFLD might have had important associations with cardiovascular outcomes in the past decade [9, 10]. Some reports have shown that MAFLD correlates more strongly with CVD than NAFLD [11, 12]. However, the link between NAFLD/MAFLD and CVD is more complex than previously thought, and it remains unclear how NAFLD/MAFLD is associated with the development of CVD [13].
Recently, a cohort study that enrolled 120,795 NAFLD patients with matched controls extracted from primary healthcare databases from four European countries reported no association between NAFLD and the risk of acute myocardial infarction (AMI) or stroke, after adjustments for established cardiovascular risk factors [14]. CVD may affect various populations differently. The incidence of organic coronary artery disease (CAD), a major cause of heart failure in Western countries, is relatively low in East Asian countries [15]. The age-adjusted death rate resulting from ischemic heart disease in Japan was estimated to be one-sixth of that in the United States [16].
Therefore, we undertook a longitudinal analysis of NAFLD/MAFLD based on prescription records derived from a large nationwide administrative claims database to estimate the incident risk of developing CVD in cohorts encountered in routine practice. The main objective of this study was to compare the incidence rate of CVD in the NAFLD and non-NAFLD groups. Furthermore, the same study was also conducted in the MAFLD group and the non-MAFLD group.
Methods
Large claims database
A large claims database constructed by the Japan Medical Data Center (JMDC Co., Ltd. Tokyo, Japan), using standardized disease classifications and anonymous record linkage [17], was used in this retrospective cohort study. This claims database contains monthly claims from medical institutions and pharmacies submitted from January 2005 to April 2020 and includes records of approximately 9.6 million insured persons, comprising mainly company employees and their family members.
This study analyzed 6.8 million persons who were registered in this database from April 2013 to March 2019. The JMDC database provides information on the beneficiaries, including encrypted personal identifiers, age, sex, data from health check-ups, questionnaire information from several insurance unions or International Classification of Diseases 10th revision (ICD-10) codes from the Ministry of Health, Labor and Welfare of Japan, diagnostic codes, and treatment fee codes. In addition, information concerning the name, dose, and number of days a prescribed drug was supplied and/or dispensed was obtained from the database. All medications were coded according to the Anatomical Therapeutic Chemical Classification of the European Pharmaceutical Market Research Association. An encrypted personal identifier was used to link claims data from different hospitals, clinics, and pharmacies. Based on the JMDC database, we defined our study cohort to enable us focus on CVD onset. The Ethics committee/institutional review board of Yokohama City University determined that ethics approval was not required because personal information in the claims database used in this study was completely deleted.
NAFLD patients and study design
Figure 1 shows the flowchart for patient selection (NAFLD subjects). People who underwent a medical check-up between April 2013 and March 2019 were selected from the JMDC claims database (n = 6,762,022). People for whom all health check-up data [body mass index (BMI), waist circumstance, sex, age, aspartate transaminase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), blood pressure, fasting blood glucose or HbA1c, and questionnaire on alcohol habits) were available within the same year and ≥ 365 days after the date of the initial observation were enrolled (n = 1,828,993). Among the patients, those with malignant neoplasms of the liver and/or intrahepatic bile ducts [ICD-10 code (ICD10): C22], viral hepatitis (ICD10: B15-19), alcoholic liver disease (ICD10: K70), primary biliary cirrhosis (ICD10: K74.3), autoimmune hepatitis (ICD10: K75.4), and excessive alcohol drinking habit (Supplementary Table 1) were excluded. Finally, 1,542,688 patients were included in this analysis.
Because the JMDC database does not contain information such as liver imaging or histology results, a definitive diagnosis of NAFLD could not be made based on database records alone. Thus, NAFLD patients were defined as patients with (1) no secondary cause of liver injury such as significant alcohol consumption, viral hepatitis, autoimmune hepatitis, primary biliary cholangitis (or primary biliary cirrhosis), or malignant neoplasm of the liver and/or intrahepatic bile ducts and (2) the presence of fatty liver was defined using the Fatty Liver Index (FLI) prediction model [18,19,20,21]. FLI was calculated using the following formula: \({\text{FLI}} = \left( {{\text{e}} ^{{0.{953}*{\text{loge }}\left( {{\text{triglycerides}}} \right) + 0.{139}*{\text{BMI}} + 0.{718}*{\text{loge }}\left( {{\text{ggt}}} \right) + 0.0{53}*{\text{waist circumference}} - {15}.{745}}} } \right)/\left( {{1} + {\text{e}} ^{{0.{953}*{\text{loge }}\left( {{\text{triglycerides}}} \right) + 0.{139}*{\text{BMI}} + 0.{718}*{\text{loge }}\left( {{\text{ggt}}} \right) + 0.0{53}*{\text{waist circumference}} - {15}.{745}}} } \right) \, \times { 1}00\) [18]. In this study, the presence of steatosis was diagnosed based on a FLI ≥ 60 that identified hepatic steatosis [21].
MAFLD patients and study design
Figure 2 shows the flowchart for patient selection of the MAFLD cohort. Patients who underwent a medical check-up between April 2013 and March 2019 were selected from the JMDC claims database (n = 6,762,022). Patients for whom all health check-up data (BMI, waist circumstance, AST, ALT, GGT, LDL-C, HDL-C, TG, blood pressure, fasting blood glucose, or HbA1c) were available within the same year and ≥ 365 days after the date of the initial observation were enrolled (n = 2,452,499). In this study, MAFLD was diagnosed based on a FLI ≥ 60 that identified hepatic steatosis [21], associated with the presence of any one of the following three metabolic conditions: diabetes mellitus, overweight/obesity, or metabolic syndrome [4]. According to the definition of MAFLD [4], overweight patients of Asian origin were defined as those with a body mass index (BMI) ≥ 23 kg/m2. Metabolic dysregulation was defined as the presence of two or more of the following conditions: (1) waist circumference ≥ 102 in men and 88 cm in women, (2) blood pressure ≥ 130/85 mmHg or specific drug treatment, (3) TG ≥ 150 mg/dl or specific drug treatment, (4) HDL-C < 40 mg/dl for men and < 50 mg/dl for women or specific drug treatment, (5) prediabetes (i.e., fasting glucose levels 100–125 mg/dl, or 2-h post-load glucose levels 140 to 199 mg/dl or HbA1c 5.7–6.4%, (6) Homeostasis model assessment of insulin resistance score ≥ 2.5, and (7) Plasma high-sensitivity C-reactive protein level > 2 mg/L [4]. Since the JMDC database does not have data on 2-h post load glucose levels, HOMA-R, and high-sensitivity C-reactive protein, these factors were not used.
Hypertriglyceridemia, hypertension, and diabetes mellitus
Hypertriglyceridemia was defined based on a prescription of anti-hypertriglyceridemia medication or a serum TG level ≥ 150 mg/dL with no anti-triglyceride medication. Hypertension was defined if the patient was receiving antihypertensive medication and ICD-10 code for hypertension (ICD10: I10), or had a systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg. DM was defined based on a prescription of antidiabetic medication or a fasting blood glucose level ≥ 126 mg/dL or a HbA1c level ≥ 6.5% with no antidiabetic medication [22] (Supplementary Table 2).
Study outcome and definitions
The primary study outcome was the incidence rate of CVD, which was defined as procedure code or the combination of ICD codes and procedure code (Supplementary Table 2). The treatment fee codes for percutaneous coronary intervention are K546, K547, K548, and K549. The treatment fee code for coronary artery bypass grafting is K552. The ICD-10 code for cerebral infarction is I63, and the treatment fee codes for cerebral infarction are K149, K164, K178, K609, and K610. CVD (cerebral infarction + coronary artery event) were measured during the follow-up period after the initiation of study recruitment in NAFLD and MAFLD patients, and compared with non-NAFLD and non-MAFLD patients, respectively. The secondary outcome was the hazard ratio of CVD with or without hypertriglyceridemia and/or DM in NAFLD and non-NAFLD patients.
Patient and public involvement
No patients were involved in the setting of this study or the analysis of the results. However, the Japanese Society of Gastroenterology has published guidelines for the public on the Internet, and the public is also becoming more interested in the relationship between NAFLD and cardiovascular disease [23].
Statistical analysis
Categorical variables are summarized as percentages, and continuous variables are summarized as means ± standard deviations. Between-group comparisons were evaluated using the Student’s t test or Chi-square test, as appropriate. Within the NAFLD and non-NAFLD groups, and MAFLD and non-MAFLD groups, we estimated the incidence rates of coronary artery events, cerebral infarctions, and CVD by dividing the number of incident events by the total number of person years at risk. The corresponding 95% confidence intervals were estimated assuming a Poisson distribution. The hazard ratios for incident coronary artery events, cerebral infarction, and CVD associated with a diagnosis of NAFLD were independently estimated using the Cox proportional hazards models. The models were adjusted by (1) age, sex, and smoking habit and (2) age, sex, smoking habit, body mass index (BMI), LDL-C, hypertension, DM, hypertriglyceridemia, and statin use in multivariable models. The hazard ratios for ischemic coronary events, cerebral infarction, and CVD events associated with a diagnosis of MAFLD were independently estimated using the Cox proportional hazards models. The models were adjusted by (1) age, sex, and smoking habit and (2) age, sex, smoking habit, LDL-C, and statin use in multivariable models. Hazard ratios for coronary artery events, cerebral infarction, and CVD are measured with or without hypertriglyceridemia and/or DM after adjusted by age, sex, smoking habit, BMI, LDL-C, hypertension, and statin use in NAFLD compared with non-NAFLD groups. Directed acyclic graph (DAG) was presented to explain our theory (supplementary Fig. 1) [24].
Results
Cohort characteristics
In total, 1,542,688 patients were included in the NAFLD cohort, while 2,452,949 patients were included in the MAFLD cohort. The prevalence of NAFLD was estimated to be 9.2% (n = 142,158), while MAFLD was estimated to be 9.7% (n = 237,242). The demographic and clinical characteristics of the NAFLD and non-NAFLD groups, and MAFLD and non-MAFLD groups are shown in Table 1. The prescription history of each group is shown in Supplemental Tables 3 and 4. Traditional cardiovascular risk factors were more common in the NAFLD and MAFLD groups than in the non-NAFLD and non-MAFLD groups. Specifically, the percentage of smokers and the male-to-female ratio were much higher in the NAFLD and MAFLD groups than in the non-NAFLD and non-MAFLD groups. Furthermore, the serum LDL-C level, serum TG level, systolic blood pressure, diastolic blood pressure, fasting blood glucose, and HbA1c level were significantly higher and the serum HDL-C level was significantly lower in the NAFLD and MAFLD groups than in the non-NAFLD and non-MAFLD groups. The overall prevalence of hypertriglyceridemia, DM, and the combination of hypertriglyceridemia and DM were 13.1%, 4.2%, and 1.0%, respectively, in the non-NAFLD group and 63.6%, 20.2%, and 12.6%, respectively, in the NAFLD group (Table 1, Fig. 3a). The overall prevalence of hypertriglyceridemia, DM, and the combination of hypertriglyceridemia and DM were 13.6%, 4.3%, and 1.1%, respectively, in the non-MAFLD group and 64.1%, 20.6%, and 12.9%, respectively, in the MAFLD group (Table 1, Fig. 3b).
CVD incidence rates
The numbers of cerebral infarctions, CAD events, and CVD events were 313, 2679, and 2981, respectively, among the 1,400,530 non-NAFLD patients in 4.0 years and 41, 831, and 868, respectively, among the 142,158 NAFLD patients in 4.0 years. The incidence rates of CAD were 0.87 (95% CI 0.84–0.91) and 2.70 (95% CI 2.52–2.89) per 1000 person-years in the non-NAFLD and NAFLD groups, respectively. The incidence rates of CVD were 0.97 (95% CI 0.94–1.01) and 2.82 (95% CI 2.64–3.01) per 1000 person-years in the non-NAFLD and NAFLD groups, respectively. The incidence rates of cerebral infarction were 0.10 (95% CI 0.09–0.11) per 1000 person-years and 0.13 (95% CI 0.10–0.18) per 1000 person-years in the non-NAFLD and NAFLD groups, respectively (Table 2).
The numbers of cerebral infarctions, CAD events, and CVD events were 563, 4675, and 5217, respectively, among the 2,215,707 non-MAFLD patients in 4.0 years and 81, 1423, and 1498, respectively, among the 237,242 MAFLD patients in 4.0 years. The incidence of CAD was 0.90 (95% CI 0.88–0.93) and 2.55 (95% CI 2.42–2.69) per 1000 person-years in the non-MAFLD and MAFLD groups, respectively. The incidence rates of CVD were 1.01 (95% CI 0.98–1.03) and 2.69 (95% CI 2.55–2.83) per 1000 person-years in the non-MAFLD and MAFLD groups, respectively. The incidence rates of cerebral infarction were 0.11 [95% CI 0.10–0.12] per 1000 person-years and 0.14 [95% CI 0.12–0.18] per 1000 person-years in the non-MAFLD and MAFLD groups, respectively (Table 2).
Hazard ratio for CVD events
Without adjustments, the respective hazard ratios for cerebral infarction, CAD, and CVD were 1.30 (95% CI 0.94–1.80), 3.08 (2.85–3.33), and 2.89 (2.68–3.12) in the NAFLD group compared to the non-NAFLD group. When adjustments were made for age, sex, and smoking habit, the hazard ratios for cerebral infarction, CAD, and CVD were 1.32 (95% CI 0.95–1.83), 2.70 (2.50–2.92), and 2.56 (2.37–2.77), respectively. However, when adjustments were made for age, sex, smoking habit, BMI, LDL-C, existence of high blood pressure, existence of DM, existence of hypertriglyceridemia, and statin use, the respective hazard ratios for cerebral infarction, CAD, and CVD were 0.96 (95% CI 0.63–1.48), 1.01 (0.91–1.13), and 1.02 (0.92–1.14) in the NAFLD group compared to the non-NAFLD group (Fig. 4A).
Hazard ratio for primary outcomes (A) NAFLD patients. ●: unadjusted. ▲: adjusted by age, sex, and smoking habit. ■: adjusted by age, sex, smoking habit, body mass index, low density lipoprotein cholesterol, hypertension, diabetes, hypertriglyceridemia, and statin use. Hazard ratios in NAFLD and non-NAFLD patients. a Cerebral infarction, b coronary artery event, and c cardiovascular event. B MAFLD patients. ●: unadjusted. ▲: adjusted by age, sex, and smoking habit. ■: adjusted by age, sex, smoking habit, low density lipoprotein cholesterol, and statin use. Hazard ratios in MAFLD and non-MAFLD patients. Bars indicate 95% confidence intervals. NAFLD: non-alcoholic fatty liver disease, MAFLD: metabolic dysfunction associated fatty liver disease. a Cerebral infarction, b coronary artery event, and c cardiovascular event
Without adjustments, the respective hazard ratios for cerebral infarction, CAD, and CVD were 1.33 (95% CI 1.06–1.68), 2.82 (95% CI 2.66–2.99), and 2.66 (95% CI 2.51–2.82), respectively, in the MAFLD group compared to the non-MAFLD group. When adjustments were made for age, sex, and smoking habit, the hazard ratios for cerebral infarction, CAD, and CVD were 1.33 (95% CI 1.04–1.68), 2.43 (2.29–2.58), and 2.33 (2.19–2.46), respectively. Furthermore, when adjustments were made for age, sex, smoking habit, LDL-C, and statin use, the respective hazard ratios for cerebral infarction, CAD, and CVD were 1.25 (95% CI 0.98–1.59), 1.98 (1.86–2.10), and 1.89 (1.78–2.01) in the MAFLD group compared to the non-MAFLD group (Fig. 4B).
Hazard ratios for CVD events in NAFLD patients with or without hypertriglyceridemia and/or diabetes
When adjustments were made for age, sex, smoking habit, BMI, LDL-C, existence of hypertension, and use of statin, the respective hazard rations for cerebral infarction were 0.43 (0.18–1.04), 1.82 (0.69–4.79), and 1.53 (0.65–3.61) in the presence of hypertriglyceridemia only, DM only, or both hypertriglyceridemia and DM, respectively, than in the absence of either DM or hypertriglyceridemia in the NAFLD groups (Fig. 5A).
Hazard ratios of primary outcomes in NAFLD patients with or without diabetes and/or hypertriglyceridemia. A Cerebral infarction, B coronary artery event, and C cardiovascular event. Primary outcomes were adjusted by age, sex, smoking habit, body mass index, low density lipoprotein cholesterol hypertension, and statin use. Bars indicates 95% confidence intervals. HR hazard ratio, NAFLD non-alcoholic fatty liver disease
When adjustments were made for the factors mentioned above, the respective hazard ratios for CAD were 1.38 (1.11–1.71), 1.75 (1.33–2.30), and 2.87 (2.30–3.58) in the presence of hypertriglyceridemia only, DM only, or both hypertriglyceridemia and DM, respectively, than in the absence of either DM or hypertriglyceridemia in the NAFLD groups (Fig. 5B).
Finally, the respective hazard ratios for CVD events in the presence of hypertriglyceridemia only, DM only, and both hypertriglyceridemia and DM were 1.31 (1.06–1.61), 1.74 (1.33–2.26), and 2.77 (2.24–3.43) in the NAFLD group after adjustments with similar factors (Fig. 5C).
Discussion
This study is a cohort study conducted to verify the correlation between fatty liver defined from two different points of view: NAFLD and MAFLD, and the risk of CVD. In the present study, the incidence rates of cerebral infarction, CAD, and CVD were 0.13 (95% CI 0.10–0.18), 2.70 (2.52–2.89) and 2.82 (2.64–3.01) per 1000 person-years, respectively, in the NAFLD group. Alexander et al. reported that the incidence rates of stroke and AMI were 4.40 (4.22–4.59) and 2.07 (1.94–2.20) per 1000 person years, respectively, in the NAFLD group [14]. The difference in CVD risk between our report and previous reports might be due to regional differences [15, 16] and the proportion of elderly individuals. A representative epidemiological study in Japan (Takashima Cardio-cerebrovascular Disease Registration System and Suita study) reported that the incidence of coronary events was 1.0–2.8/1000 person-years, which is very close to the results of this study [25,26,27].
When adjustments were made for age, sex, and smoking habit, the hazard ratio for CVD was 2.56 in the NAFLD patients compared to the non-NAFLD patients. However, after adjustments for metabolic syndrome factors, LDL-C, and statin use, the CVD risk was almost the same (HR: 1.02) in the NAFLD and non-NAFLD groups, which is consistent with the report by Alexander et al. using a European database [14]. In this study, we also investigated the CVD risk associated with MAFLD. In 2020, an international expert panel proposed a new disease concept, "metabolic dysfunction associated fatty liver disease (MAFLD)," in which fatty liver caused by nutritional metabolic disorders can coexist with other chronic liver diseases [4]. In the MAFLD group of our study, the risk of CAD and CVD was higher than in non-MAFLD, even after adjusting by factors other than the MAFLD definition components that are associated with CVD such as age, gender, smoking status, LDL-C level, and statin use. Recently, there have been recent reports of cross-sectional observations or cohort studies showing an association between MAFLD and CVD, and in both cases, the risk ratios for CVD are higher in MAFLD populations than in NAFLD populations [12, 28, 29].
Further, we focused on DM and hypertriglyceridemia, which are important risk factors for both NAFLD, MAFLD and CVD. The role of lipogenesis in NAFLD and MAFLD development is important [18]. The accumulation of fat, mainly TG, within hepatocytes is a prerequisite for NAFLD/MAFLD development. The blockage of very-low-density lipoprotein-TG secretion, which can be caused by a dietary choline deficiency, and a reduction in fatty acid oxidation occur in the pathogenesis of nonalcoholic steatohepatitis (NASH) [30]. NAFLD, MAFLD and metabolic syndrome share many traditional, but not strictly hepatospecific, pathophysiological mechanisms. The prevalence of complications of hypertriglyceridemia only, DM only, and both hypertriglyceridemia and DM was much higher in the NAFLD and MAFLD group than in the non-NAFLD and non-MAFLD group. The risk of CVD event increased by the complication of hypertriglyceridemia and DM in the NAFLD. Additionally, combining the risk of hypertriglyceridemia and DM increased the CVD risk by about threefold in both the NAFLD groups.
Approximately 9.2% of the population (142,158/1,542,688) and 9.7% of the population (237,242/2,452,949) in this study were considered to have clinical features of NAFLD and MAFLD, respectively. Traditional cardiovascular risk factors, such as smoking habit, male sex, BMI, dyslipidemia, hypertension, and DM, were more common in the participants with NAFLD and MAFLD than in the non-NAFLD and non-MAFLD group. Previously, three cohort studies that involved 3,000–5,000 subjects, who underwent health check-ups, reported a NAFLD prevalence of 18.0–29.7% in Japan [31,32,33]. Additionally, Li et al. reported that the prevalence of NAFLD was 22.3% in Japan based on a meta-analysis [2]. The incidence of MAFLD has not yet been reported in detail. People who undergo annual health check-ups, included in the JMDC database, have between 20 and 60 years. Therefore, it is assumed that the incidence of NAFLD and MAFLD in this study was lower than it actually is.
The prevalence of NAFLD and NASH, and possibly of MAFLD, has increased rapidly worldwide, along with increases in the prevalence of obesity and DM [1, 2]. Currently, NAFLD/MAFLD represents one of the most important global health problems from a medical and socioeconomic standpoint. Recently, a growing body of evidence was collected to support the notion that NAFLD is both a liver specific disease and an early mediator of systemic diseases. The present retrospective longitudinal observational cohort study evaluated the incidence of CVD in 1.6—2.5 million subjects in Japan for about 4 years based on nationwide prescription records from a large administrative claims database. Real-world evidence including non-interventional studies, patient registries, claims database studies, patient surveys, and electronic health record studies, if appropriately designed, can provide valuable information concerning practice patterns and patient characteristics in actual clinical settings. The strengths of our study were the large sample size and the precise definition of CVD based on data from medical practice [34], which allowed us to accurately identify almost all patients who developed CVD during the follow-up period.
This study had several limitations. First, the JMDC Claims Database is an epidemiological receipt database that has accumulated receipts (inpatient, outpatient, dispensing) and medical examination data from several health insurance providers; therefore, the data of individuals aged under 20 years and of those aged over 65 years may be insufficient. Second, we investigated the risk of CVD by dividing the subjects into groups based on FLI, not by imaging or histopathological information. However, the use of highly objective markers such as FLI has been considered appropriate for conducting large epidemiological studies [4, 21]. FLI is thought be an important tool in epidemiological studies, particularly when assessing the incidence of NAFLD [35]. On the other hand, FLI is rarely measured in daily practice, and more than 80% of liver diseases in which other liver diseases have been ruled out are NAFLD. When classified by the presence or absence of ALT abnormalities (33 IU/L for men and 25 IU/L for women) [36], CVD was predominantly higher in the consist with NAFLD than in the non-NAFLD group (Supplementary Fig. 2 and 3, Supplementary table 5). Third, we excluded alcoholic liver disease using the ICD-10 and the results of a questionnaire to detect alcohol habits and amounts in the NAFLD study. However, the possibility of alcoholic liver disease cannot be completely excluded. Fourth, the diagnostic criteria for MAFLD are still at the stage of recommendation by the expert panel, and may change in the future. In addition, there are some items that have not been examined in this study, such as high-sensitivity CRP, HOMA-IR, and glucose tolerance test result. Finally, the JMDC Claims Database did not contain information on hepatic fibrosis. Several studies have indicated that hepatic fibrosis contributes to atherogenesis [37, 38]. Estes et al. reported that there are 3.76 million NASH patients in Japan (3% of the country’s population). Of these patients, 0.67 million people had F3/F4 fibrosis [39]. Patients with fibrosing NASH may develop CVD events and undergo an acceleration of atherosclerosis, possibly via increased hepatic production of several prothrombogenic factors, such as vascular endothelial growth factor, hypoxia-inducible factor, intracellular adhesion molecule-1, vascular adhesion molecule-1, and fetuin-A [13]. Study results may vary depending on the number of NASH cases with advanced fibrosis.
NAFLD and MAFLD are regarded as the liver component of metabolic syndrome and is reportedly associated with risk factors for metabolic syndrome. All the factors potentially involved in atherosclerotic processes are related to NAFLD and MAFLD [13, 40,41,42,43]. As discussed previously, regional differences in CVD prevalence exist worldwide [15, 16]. However, CVD is the most common cause of death in NAFLD, and possible in MAFLD patients not only in the United States and Europe, but also in Japan [44, 45]. Our study shows that patients with NAFLD and MAFLD have a higher risk of CVD.
Data availability
No additional data available.
Abbreviations
- AMI:
-
Acute myocardial infarction
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transaminase
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- HDL-C:
-
High-density lipoprotein cholesterol
- ICD-10:
-
International classification of diseases 10th revision
- JMDC:
-
Japan medical data center
- LDL-C:
-
Low-density lipoprotein cholesterol
- MAFLD:
-
Metabolic dysfunction associated fatty liver disease
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- TG:
-
Triglyceride
- DM:
-
Diabetes mellitus
References
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–98.
Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–10.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9.
Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389-97.e10.
Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–73.
Park HE, Lee H, Choi SY, et al. Clinical significance of hepatic steatosis according to coronary plaque morphology: assessment using controlled attenuation parameter. J Gastroenterol. 2019;54:271–80.
Lee YH, Kim KJ, Yoo ME, et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68:764–72.
Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50.
Bhatia LS, Curzen NP, Calder PC, et al. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190–200.
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2020;S1542–3565(20):31717–21.
Tsutsumi T, Eslam M, Kawaguchi T, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res. 2021 (Online ahead of print)
Lonardo A, Nascimbeni F, Mantovani A. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–52.
Alexander M, Loomis AK, van der Lei J, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367.
Sasayama S. Heart disease in Asia. Circulation. 2008;118:2669–71.
Baba S, Ozawa H, Sakai Y, et al. Heart disease death in Japanese urban area evaluated by clinical and police records. Circulation. 1994;89:109–15.
Kimura S, Sato T, Ikeda S, et al. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20:413–9.
Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity. EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–5715.4 Wong VW JGH 2018;33:70–85.
Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85.
American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11-66.
Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364–77.
Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package “dagitty.” Int J Epidemiol. 2016;45:1887–94.
Iso H. Changes in coronary heart disease risk among Japanese. Circulation. 2008;118:2725–9.
Rumana N, Kita Y, Turin TC, et al. Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI Registry, 1990–2001. Am J Epidemiol. 2008;167:1358–64.
Nishimura K, Okamura T, Watanabe M, et al. Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: the suita study. J Atheroscler Thromb. 2014;21:784–98.
Guerreiro GTS, Longo L, Fonseca MA, et al. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int. 2021 [Online ahead of print].
Guerreiro GTS, Longo L, Fonseca MA, et al. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int. 2021;15:380–91.
Fujita K, Nozaki Y, Wada K, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–80.
Omagari K, Kadokawa Y, Masuda J, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17:1098–105.
Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8.
Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–95.
Yamada MH, Fujihara K, Kodama S, et al. Associations of systolic blood pressure and diastolic blood pressure with the incidence of coronary artery disease or cerebrovascular disease according to glucose status. Diabetes Care. 2021 [Online ahead of print].
Vanni E, Bugianesi E. Editorial: utility and pitfalls of Fatty Liver Index in epidemiologic studies for the diagnosis of NAFLD. Aliment Pharmacol Ther. 2015;41:406–7.
Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35.
Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600.
Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65.
Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904.
Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metab Clin Exp. 2016;65:1136–50.
Rifai M, Silverman MG, Nasir K, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239:629–33.
Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306–24.
Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–71.
Tada T, Kumada T, Toyoda H, et al. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:899–910.
Akuta N, Kawamura Y, Arase Y, et al. Hepatocellular carcinoma is the most common liver-related complication in patients with histopathologically-confirmed NAFLD in Japan. BMC Gastroenterol. 2018;18:165.
Acknowledgements
The authors would like to thank Kowa Company. Ltd. Who provided funds to JMDC Inc. for analysis of the data (grant ID: 191203KOWA). All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: TY is an employee of Kowa Company, Ltd. and AN received research supports from Kowa Company, Ltd; no other relationships or activities have influenced the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Masato Yoneda, Takuma Yamamoto, and Atsushi Nakajima. The first draft of the manuscript was written by Masato Yoneda, Takuma Yamamoto, and Atsushi Nakajima, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoneda, M., Yamamoto, T., Honda, Y. et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol 56, 1022–1032 (2021). https://doi.org/10.1007/s00535-021-01828-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-021-01828-6