Abstract
Anemia is a common complication of chronic kidney disease (CKD) in children, and dysregulation of iron homeostasis plays a central role in its pathogenesis. Optimizing iron status is a prerequisite for effective treatment of anemia. Insufficient iron can lead to inappropriate escalation of the erythropoiesis-stimulating agent (ESA) dose, which is associated with adverse outcomes. Excess iron supplementation also has negative sequelae including free radical tissue damage and increased risk of systemic infection. Notwithstanding the importance of optimizing bioavailable iron for erythropoiesis for children with advanced CKD, achieving this remains challenging for pediatric nephrologists due to the historical lack of practical and robust measures of iron status. In recent years, novel techniques have come to the fore to facilitate accurate and practical assessment of iron balance. These measures are the focus of this review, with emphasis on their relevance to the pediatric CKD population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is a common complication of advanced chronic kidney disease (CKD), with prevalence exceeding 87% in children with CKD stages 4 and 5 [1, 2]. The pathogenesis and management of anemia in CKD were recently reviewed elsewhere [3]. Deficiency and dysregulation of iron play a central role [1, 3, 4].
Optimizing iron status is a prerequisite for effective treatment of anemia in children with CKD. If sufficient iron is not available for erythropoiesis, this can lead to inappropriate escalation of erythropoiesis-stimulating agent (ESA) therapy, which is associated with adverse outcomes in both adults and children [5,6,7,8]. Conversely, iron overload also has negative sequelae including free radical tissue damage, increased risk of systemic infection, and more hospitalizations [9,10,11]. Achieving the optimal balance of bioavailable iron for erythropoiesis in children with advanced CKD is therefore paramount in anemia management; however, it remains challenging for pediatric nephrologists. A key reason for this is the historical lack of practical yet robust measures of iron status. In recent years, novel techniques have come to the fore that can help to facilitate accurate and practical assessment of iron balance. These measures are the focus of this review.
Iron homeostasis in health
A working knowledge of iron homeostasis in healthy children is helpful background for considering measures of iron status and their application to anemia management in CKD. The key processes responsible for regulation of iron absorption and release are reviewed in detail elsewhere [12, 13] and briefly summarized here.
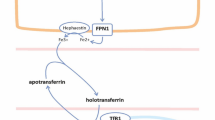
Approximately 75% of the body’s iron circulates in erythrocytes, with a smaller proportion stored in hepatocytes for release when required. Less than 0.1% total body iron circulates in plasma bound to transferrin, its main transport protein. In health, daily iron intake and losses comprise just 0.05% total body iron and are tightly balanced via feedback control. Iron in erythrocytes is efficiently recycled via the reticuloendothelial system; macrophages phagocytose senescent erythrocytes and release controlled quantities of iron into the circulation, which is transported to the bone marrow via transferrin for incorporation into red cell precursors (Fig. 1).
Iron recycling via the reticuloendothelial system. Approximate proportion of body iron stores in each system are indicated in parentheses. Iron measures are depicted in red text. TSAT transferrin saturation, CHr reticulocyte hemoglobin content, Ret-He reticulocyte hemoglobin equivalent, %HRC proportion of hypochromic red cells
Circulating iron levels are tightly regulated. Both import of iron via duodenal enterocytes to plasma and the release of recycled iron from macrophages and stored iron from hepatocytes to plasma are mediated by the cellular transport protein ferroportin [14]. Ferroportin expression is controlled by hepcidin, the hormone chiefly responsible for extracellular iron regulation. Hepcidin negatively regulates plasma iron levels by binding to ferroportin and inducing its internalization [15].
Although iron stores and bioavailable iron in plasma are tightly regulated in health, iron homeostasis is disturbed in CKD. The underlying reasons and their relevance to iron measurements will now be outlined.
Iron dysregulation in CKD
Iron deficiency in children with CKD can be thought of as either absolute, functional, or a combination of both. Absolute iron deficiency refers to insufficient body iron stores. Functional deficiency refers to a situation in which the bioavailability of iron for incorporation into reticulocytes is compromised, despite normal or excessive total body iron stores.
Absolute iron deficiency in CKD can result from insufficient dietary iron intake or duodenal iron absorption, and/or from excessive iron loss, for example through extracorporeal hemodialysis circuits or gastrointestinal sloughing. Functional iron deficiency results from impaired extracellular iron homeostasis. In recent years, hepcidin dysregulation has been found to play a key role in the functional iron deficiency in CKD. Hepcidin is a 2.7-kDa protein which is filtered by the kidneys [16]. Hepcidin clearance is therefore reduced in CKD disrupting its regulation [17]. A further reason for inappropriate elevation in hepcidin is chronic inflammation, a well-recognized complication of CKD [18]. The net effect of reduced renal clearance, and inflammatory upregulation, is chronic inappropriate elevation in hepcidin concentration resulting in suppression of both duodenal absorption of iron and its release from macrophages and hepatocytes.
The combined effect of reduced iron absorption and release results in a net reduction in iron available for incorporation into emerging reticulocytes in the bone marrow, resulting in iron-restricted erythropoiesis. This results in a suboptimal response to ESA, which can lead to inappropriate escalation of ESA doses if suitable assessment of bioavailable iron is not undertaken to inform iron supplementation.
Aims of iron measurement
Optimizing iron balance in children with CKD involves ensuring availability of sufficient iron for red cell precursors to use as raw material for erythropoiesis, while avoiding excessive iron supplementation and its sequelae. Measures of iron status should therefore indicate the amount of bioavailable iron to red blood cell precursors for incorporation into hemoglobin; they should also indicate iron excess. Minimizing blood sampling is also an important consideration in children, since frequent sampling can exacerbate iron depletion, particularly in infants for whom blood samples constitute a greater proportion of iron stores. Cost is another important factor in healthcare systems that are increasingly budget constrained. Acceptable levels of variability, both biological and analytical, are also key requirements for a robust measure of iron status.
A key challenge in developing suitable measures of iron in patients with CKD is the lack of an established gold standard comparator. Previously, iron staining of bone marrow biopsy specimens was considered the gold standard for assessment of iron stores. This is unsuitable for children with CKD for a number of reasons. Firstly, bone marrow iron staining is not a robust test; it has significant variability and is dependent on the operator and process used. Secondly, sampling is a painful procedure that requires a general anesthetic in the majority of children. Thirdly, bone marrow biopsy is impractical to repeat on a regular basis as would be required for iron monitoring in children on dialysis. Due to the impracticality of assessing bone marrow iron stores, studies on adult patients have used clinical parameters as gold standard markers of iron repletion, most commonly the response in hemoglobin to ESA. This is currently the most clinically relevant and practical standard for evaluating iron measures in children with CKD.
In summary, iron measures for children with CKD should reliably quantify bioavailable iron for erythropoiesis as reflected in clinical erythropoietic response to ESA. Traditional and novel iron measures will now be discussed with consideration of these requirements.
Limitations of traditional iron measures
Traditionally, a combination of transferrin saturation (TSAT) and serum ferritin was used to assess iron status in adults and children with CKD, and these measures are still recommended in The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease: Improving Global Outcomes (KDIGO) anemia guidelines [19, 20]. There are however significant limitations to both measures that compromise their clinical utility.
Biological and analytical variability
Biological variability refers to differences in the measured parameter between patients, and in the same patient measured at different times, resulting from physiological variation in a short period of time. Analytical variability refers to differences in test results performed on the same sample resulting from variability in the testing process.
Serum ferritin and TSAT have been shown to exhibit a high degree of both analytical and biological variability in adult patients with end-stage kidney disease (ESKD) [21, 22]. This is one of the key reasons underlying the recommendation by the National Institute for Health and Care Excellence not to use these parameters alone to assess iron status in CKD [23].
Inflammation confounds measurements
Both serum ferritin and TSAT measurements are confounded by inflammation. Chronic inflammation is a feature of CKD in both adults and children and is partly related to suboptimal nutrition and dialysis [24,25,26]. Infections are a further source of inflammation and are a particular issue in children with central venous catheters or peritoneal dialysis access [27, 28]. Inflammation in children with CKD and ESKD compromises the reliability of serum ferritin and TSAT to reliably reflect patients’ iron status.
Ferritin is an intracellular iron storage protein. Its concentration in serum is influenced by several factors, one of which is intracellular iron stores. Serum ferritin is also a marker of acute inflammation and has a key role in the diagnosis of systemic inflammatory processes such as macrophage activation syndrome [29]. Serum ferritin is also affected by patients’ nutritional status as observed in adult hemodialysis patients [30]. Given significant confounding influences, the utility of ferritin as a marker of iron status in patients with kidney disease has been questioned for over a decade [31].
Transferrin saturation is not itself measured, but rather derived from measurements of serum iron and total iron binding capacity (TIBC). TIBC is a negative acute-phase reactant, that is, its plasma concentration is suppressed by inflammation. Low TIBC levels are associated with increased mortality in adult patients on dialysis [32, 33]. In the context of systemic inflammation, reductions in TIBC lead to higher levels of TSAT independent of patients’ iron status. Inflammation is therefore implicated in the poor reliability of TSAT as a measure of iron status in CKD.
Comorbidity confounds measurements
Malnutrition is fortunately uncommon in pediatric nephrology units with adequate dietetic support; however, it is clearly associated with elevated serum ferritin levels in adults with CKD independent of iron status [30].
Liver disease also confounds serum ferritin measurements. The release of ferritin from intracellular stores into the circulation is incompletely understood; however, its clearance from the circulation depends largely on the reticuloendothelial system, of which the liver is a key part. In the context of liver dysfunction, e.g., due to hepatitis or fatty liver disease, reticuloendothelial clearance of circulating ferritin is impaired, resulting in higher serum ferritin levels. This is independent of iron status and therefore confounds the assessment of iron status in patients with liver disease.
Malignancy affects serum ferritin levels to such an extent that they are used as a tumor marker for both neuroblastoma and Hodgkin’s disease in children [34,35,36]. For this reason, it cannot be used to assess iron status in patients with malignancy.
Given the numerous confounding influences on both serum ferritin and TSAT in children with CKD and ESKD, the reliability of these traditional measures of iron status is poor. Alternative measures aimed at reliably reflecting bioavailable iron for erythropoiesis will now be discussed.
Alternative measures of iron status
A number of measures to estimate bioavailable iron for incorporation into emerging erythrocytes have been developed in recent years. They are illustrated in Fig. 1 and outlined here.
Reticulocyte hemoglobin content and reticulocyte hemoglobin equivalent
The application of automated blood analyzers to measure the iron content of circulating red cells to improve assessment of iron status was suggested by Macdougall over 25 years ago [37]. Subsequently, parameters which reflect availability of iron to developing red cells in the short term have been developed. Reticulocyte hemoglobin content is a measure introduced in Siemens blood count analyzers that quantify hemoglobin mass in reticulocytes. Given that hemoglobin production is directly dependent on bioavailable iron, and that reticulocytes mature into erythrocytes within days, the hemoglobin content of emerging erythrocytes reflects short-term changes in iron availability for erythropoiesis.
A related measure to assess iron bioavailability for emerging erythrocytes is the reticulocyte hemoglobin equivalent, which is available in Sysmex blood analyzers. Automated cell counts are undertaken using fluorescent markers to identify cellular RNA. Red blood cells, reticulocytes, and platelets are identified, and reticulocyte hemoglobin equivalent calculated from a combination of forward scatter and fluorescence.
Reticulocyte hemoglobin content (CHr) and reticulocyte hemoglobin equivalent (Ret-He) are both physiologically appropriate markers of iron status for children with CKD since they measure a short half-life product whose synthesis is highly dependent on iron availability. Additional advantages include less blood sampling (analysis is performed on the same EDTA sample used for full blood count analysis) and reduced cost compared to traditional measures (74% cost saving in our center).
Several studies in adult patients, and a small number of pediatric studies, support the use of CHr or Ret-He in the assessment of iron status in CKD. Their biological and analytical variability is superior to traditional iron measures [21]. In a study on 78 adult patients undergoing bone marrow examination, CHr and traditional measures of iron were compared to bone marrow iron staining as a gold standard measure of iron deficiency; receiver operating curves showed improved diagnostic performance of CHr [38]. CHr and Ret-He were superior to conventional iron measures in detecting iron-deficient erythropoiesis defined by erthropoietic response to intravenous iron therapy in studies on adult hemodialysis patients [39, 40]. Randomized controlled trials of CHr compared to TSAT showed decreased intravenous iron use with no significant difference in ESA doses when CHr was used to assess iron status [41, 42]. Ret-He was found to be both sensitive and specific in distinguishing iron deficiency anemia from other causes in adults with various pathologies [43].
A number of small pediatric studies have evaluated the utility of CHr as a maker of iron status. CHr was found to be a suitable marker of latent iron deficiency in preterm and very low birthweight infants; however, a key limitation was the use of traditional extracellular markers of iron as the gold standard comparator [44]. Ret-He was found to be a useful predictor of response to oral iron supplementation in 34 children with normal kidney function [45]. A study evaluating CHr in children with inflammatory bowel disease was inconclusive as traditional markers were used as a comparator, which are confounded by inflammation [46]. In a retrospective analysis of children on chronic dialysis, CHr was compared to traditional markers of iron status with the conclusion that prospective studies for further evaluation are warranted [47].
Pediatric reference ranges for CHr and Ret-He have been established [48, 49]. There is now sufficient evidence for the clinical utility of CHr and Ret-He that their use is recommended for the assessment of iron status in patients with CKD in European best practice guidelines [50] and UK NICE guidelines on the anemia in CKD [23].
Proportion of hypochromic red cells
The proportion of hypochromic red cells, defined as cells with hemoglobin concentration < 280 g/L, was proposed as a marker of functional iron deficiency over 25 years ago [37]. Reduction in mean cell hemoglobin results after several weeks of insufficient hemoglobin production as a result of inadequate iron supply.
In adult patients on hemodialysis, proportion of hypochromic red cells (%HRC) performed better than traditional measures in a study performed before widespread availability of reticulocyte markers [51]. %HRC is now a well-established measure of functional iron deficiency, although it is not suitable for assessing short-term changes in iron status [52]. Given the published evidence for the clinical utility of %HRC, this measure is recommended in the UK NICE guideline for anemia management in people with CKD [23].
Red blood cell size factor
The red blood cell size factor is the geometric mean of the mean cell volumes of mature red blood cells and reticulocytes. It was evaluated in healthy adults and those with anemia from various causes and found to correlate well with CHr with potential clinical utility in the diagnosis of iron-restricted erythropoiesis [53]. Published data in patients with CKD are lacking; it is therefore not currently recommended for clinical assessment of iron status in patients with kidney disease.
Soluble transferrin receptor
The transferrin receptor is expressed on red cell precursors. It facilitates the incorporation of circulating transferrin-bound iron into erythrocytes. Elevated soluble transferrin receptor levels in plasma are associated with iron deficiency [54]. Limitations of soluble transferrin receptor as a marker of iron status include confounding by the use of ESAs, cost, and lack of widespread availability [52].
Studies of the use of soluble transferrin receptor to detect iron deficiency in adult patients with CKD have shown variable results, but in general, it was found to be an inferior marker to cellular measures such as %HRC or CHr [51, 55]. Combining soluble transferrin receptor with ferritin concentration in the soluble transferrin receptor index improves its performance; however, it remains inferior to cellular markers [56].
Hepcidin
As outlined above, hepcidin is a protein synthesized by the liver that regulates both absorption of iron from the intestine and release of intracellular iron into plasma from reticuloendothelial cells following recycling of erythrocytes [12]. Expression of hepcidin is upregulated in iron overload and inflammation and downregulated in iron deficiency.
Early studies have evaluated the utility of hepcidin levels as a measure of functional iron deficiency in CKD. In a study of 78 adults with CKD stages 3 to 4, lower hepcidin levels were predictive of erythropoietic response to intravenous iron supplementation, albeit with sensitivity 84% and specificity of only 48% [57]. Clinical data and availability of hepcidin measurement are however limited, and it remains a research tool to date.
Zinc protoporphyrin
Zinc protoporphyrin is a by-product of heme synthesis, and its concentration in red cells increases when iron supply to emerging reticulocytes is reduced [58]. It has therefore been evaluated as a potential measure of bioavailable iron for erythropoiesis. Key limitations of zinc protoporphyrin include confounding of results by hyperbilirubinemia, reduced kidney function, and severe anemia [59]. These can be partly mitigated by washing red cells prior to testing, although this is expensive and not entirely practical for a routine clinical test.
Studies in adult patients evaluating the clinical utility of zinc protoporphyrin as a measure of iron status have conflicting results. While it has been reported to be predictive of iron deficiency in hemodialysis patients [60, 61], further studies found that it did not change after intravenous iron treatment and did not correlate with bone marrow iron stores [62, 63]. It is therefore not recommended in the routine clinical assessment of iron status in patients with CKD.
Clinical guidelines
National and international guidelines vary in their recommendations for iron measurement in anemia of CKD as summarized in Table 1. Prior to 2004, serum ferritin and TSAT were recommended in all guidelines and used globally to assess iron status. Since then, European, NICE, and British Committee for Standards in Haematology guidelines endorse alternative measures of %HRC, CHr, and Ret-He. KDOQI and KDIGO guidelines still recommend ferritin and TSAT, which predominate in US clinical practice.
The geographical variation in guidelines and clinical adoption of cellular measures of iron status may be due to a key limitation of these measures, namely confounding with sample storage. Measuring %HRC is unreliable if blood samples are not processed within 6 h, because cells swell during storage. Use of national laboratories by most of the large dialysis chains in the USA incurs a processing delay of 18 to 24 h due to sample shipping [64]. This delay confounds CHr, Ret-He, and %HRC measures and is a key factor hindering their adoption. Implementation of these measures is more straightforward in Europe where processing delays are minimized by using local laboratories.
Special situations
There are certain situations in which iron measures need to be interpreted with caution:
Red cell disorders such as thalassemia and glucose-6-phosphate dehydrogenase (G6PD) deficiency confound cellular measures such as CHr, Ret-He, %HRC, and red blood cell size factor. In these conditions, atypical features of both hemoglobin and red cells render cellular indices non-interpretable. Zinc protoporphyrin is also elevated in thalassemia traits and is therefore not useful for iron assessment in patients with hemoglobinopathies.
Iron measures should be also interpreted with caution in the postnatal period, as the effect of changing hemoglobin composition from HbF to HbA on cellular measures of iron has not been studied. We are currently gathering data with the aim of establishing a reference range for Ret-He in infants under 6 months of age.
Caution is needed if patients’ iron status is assessed following transfusion of packed red blood cells, which deliver a bolus of 220–250 mg iron per adult unit transfused [65]. Measurement of %HRC is confounded by red cell transfusion because infused exogenous normochromic red cells artificially reduce the proportion of circulating hypochromic cells. CHr and Ret-He are less affected by transfusion of mature erythrocytes; however, assessment of iron status is rarely helpful immediately following red cell transfusion because of the associated iron bolus.
Summary
Careful assessment of iron availability for erythropoiesis is important for children with CKD in order to optimize anemia management and avoid sequelae of iron deficiency (such as morbidity from excessive ESA doses), and excessive iron (organ toxicity and increased infection). Traditional measures of iron, serum ferritin and TSAT, are not fit for this purpose due to unacceptable analytical and biological variability, and confounding by inflammation, nutritional status, and other co-morbidities. Novel measures such as %HRC, CHr, and Ret-He offer superior assessment with additional advantages of reduced blood sampling and cost saving and are now recommended in European and UK NICE guidelines for assessment of anemia in children with CKD.
Notwithstanding these advances, no single parameter offers comprehensive assessment of body iron stores and bioavailable iron for erythropoiesis. Caution is required when interpreting iron measures in all babies in the postnatal period, and in children with erythrocyte disorders such as thalassemia. The absorption, storage, recycling, and transport of iron involve multiple interrelated control mechanisms. Looking ahead, multi-parameter algorithms incorporating patients’ age, clinical features, and a panel of iron-related measures will be desirable to further enhance the assessment of iron status in children with CKD.
Multiple choice questions (answers are provided following the reference list)
-
1.
Key determinants of serum ferritin include:
-
a)
Intracellular iron stores
-
b)
Nutritional status
-
c)
Inflammatory status
-
d)
Macrophage activity
-
e)
All of the above
-
a)
-
2.
UK NICE guidance for assessment and management of anemia in people with CKD recommends measurement of iron status with:
-
a)
CHr and TSAT
-
b)
Ret-He and zinc protoporphyrin
-
c)
Ferritin, TSAT, and free serum iron
-
d)
%HRC, or CHr/Ret-He if analysis of %HRC is not available within 6 h
-
e)
None of the above
-
a)
-
3.
Hepcidin mediates:
-
a)
Endocytosis of ferroportin
-
b)
Increased absorption of iron from the duodenum
-
c)
Reduced release of iron from macrophages to plasma
-
d)
Increased release of iron from hepatocyte stores
-
e)
a and c
-
a)
-
4.
Daily iron losses comprise the following proportion of body stores in health:
-
a)
< 0.1%
-
b)
0.1–1%
-
c)
1–10%
-
d)
> 10%
-
a)
-
5.
Complications of excess iron supplementation include:
-
a)
Increased risk of infection
-
b)
Mortality in adults with ESKD
-
c)
Tissue toxicity
-
d)
Gastrointestinal side effects
-
e)
All of the above
-
a)
References
Atkinson MA, Martz K, Warady BA, Neu AM (2010) Risk for anemia in pediatric chronic kidney disease patients: a report of NAPRTCS. Pediatr Nephrol 25:1699–1706
Staples AO, Wong CS, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Greenbaum LA (2009) Anemia and risk of hospitalization in pediatric chronic kidney disease. Clin J Am Soc Nephrol 4:48–56
Atkinson MA, Warady BA (2017) Anemia in chronic kidney disease. Pediatr Nephrol 33:227–238
Atkinson MA, Furth SL (2011) Anemia in children with chronic kidney disease. Nat Rev Nephrol 7:635–641
Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, Investigators C (2006) Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355:2085–2098
Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, Investigators T (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361:2019–2032
Borzych-Duzalka D, Bilginer Y, Ha IS, Bak M, Rees L, Cano F, Munarriz RL, Chua A, Pesle S, Emre S, Urzykowska A, Quiroz L, Ruscasso JD, White C, Pape L, Ramela V, Printza N, Vogel A, Kuzmanovska D, Simkova E, Muller-Wiefel DE, Sander A, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network R (2013) Management of anemia in children receiving chronic peritoneal dialysis. J Am Soc Nephrol 24:665–676
Lestz RM, Fivush BA, Atkinson MA (2014) Association of higher erythropoiesis stimulating agent dose and mortality in children on dialysis. Pediatr Nephrol 29:2021–2028
Cichota LC, Bochi GV, Tatsch E, Torbitz VD, Agnol PR, Zanardo JC, Barbisan F, da Cruz IB, Vaucher Rde A, Moresco RN (2015) Circulating double-stranded DNA in plasma of hemodialysis patients and its association with iron stores. Clin Lab 61:985–990
Nakanishi T, Kuragano T, Nanami M, Hasuike Y (2016) Iron localization and infectious disease in chronic kidney disease patients. Am J Nephrol 43:237–244
Varas J, Ramos R, Aljama P, Perez-Garcia R, Moreso F, Pinedo M, Ignacio Merello J, Stuard S, Canaud B, Martin-Malo A, Group ORD (2018) Relationships between iron dose, hospitalizations and mortality in incident haemodialysis patients: a propensity-score matched approach. Nephrol Dial Transplant 33:160–170
Ganz T (2013) Systemic iron homeostasis. Physiol Rev 93:1721–1741
Finch C (1994) Regulators of iron balance in humans. Blood 84:1697–1702
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810
Zaritsky J, Young B, Gales B, Wang HJ, Rastogi A, Westerman M, Nemeth E, Ganz T, Salusky IB (2010) Reduction of serum hepcidin by hemodialysis in pediatric and adult patients. Clin J Am Soc Nephrol 5:1010–1014
Malyszko J, Malyszko JS, Mysliwiec M (2009) Hyporesponsiveness to erythropoietin therapy in hemodialyzed patients: potential role of prohepcidin, hepcidin, and inflammation. Ren Fail 31:544–548
Kdoqi, National Kidney F (2006) KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47:S11–S145
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group (2012) KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2:279–335
Van Wyck DB, Alcorn H Jr, Gupta R (2010) Analytical and biological variation in measures of anemia and iron status in patients treated with maintenance hemodialysis. Am J Kidney Dis 56:540–546
Ford BA, Coyne DW, Eby CS, Scott MG (2009) Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney Int 75:104–110
(NICE) NIfHaCE (2015) Anaemia management in chronic kidney disease: update 2015. NICE guideline 8 https://www.nice.org.uk/guidance/ng8/chapter/1-recommendations
Silverstein DM (2009) Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 24:1445–1452
Canpolat N, Caliskan S, Sever L, Tasdemir M, Ekmekci OB, Pehlivan G, Shroff R (2013) Malnutrition and its association with inflammation and vascular disease in children on maintenance dialysis. Pediatr Nephrol 28:2149–2156
Sylvestre LC, Fonseca KP, Stinghen AE, Pereira AM, Meneses RP, Pecoits-Filho R (2007) The malnutrition and inflammation axis in pediatric patients with chronic kidney disease. Pediatr Nephrol 22:864–873
Hayes WN, Watson AR, Callaghan N, Wright E, Stefanidis CJ, European Pediatric Dialysis Working G (2012) Vascular access: choice and complications in European paediatric haemodialysis units. Pediatr Nephrol 27:999–1004
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A, Investigators S (2016) Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol 11:1590–1596
Sen ES, Steward CG, Ramanan AV (2017) Diagnosing haemophagocytic syndrome. Arch Dis Child 102:279–284
Kalantar-Zadeh K, Rodriguez RA, Humphreys MH (2004) Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 19:141–149
Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH (2006) The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1(Suppl 1):S9–S18
Kalantar-Zadeh K, Kleiner M, Dunne E, Ahern K, Nelson M, Koslowe R, Luft FC (1998) Total iron-binding capacity-estimated transferrin correlates with the nutritional subjective global assessment in hemodialysis patients. Am J Kidney Dis 31:263–272
Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD (2003) Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42:864–881
Silber JH, Evans AE, Fridman M (1991) Models to predict outcome from childhood neuroblastoma: the role of serum ferritin and tumor histology. Cancer Res 51:1426–1433
Blatt J, Huntley D, Eagon PK (1990) Synthesis of ferritin by neuroblastoma. Cancer Biochem Biophys 11:169–176
Hann HW, Lange B, Stahlhut MW, McGlynn KA (1990) Prognostic importance of serum transferrin and ferritin in childhood Hodgkin's disease. Cancer 66:313–316
Macdougall IC, Cavill I, Hulme B, Bain B, McGregor E, McKay P, Sanders E, Coles GA, Williams JD (1992) Detection of functional iron deficiency during erythropoietin treatment: a new approach. BMJ 304:225–226
Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ (2002) Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood 99:1489–1491
Chuang CL, Liu RS, Wei YH, Huang TP, Tarng DC (2003) Early prediction of response to intravenous iron supplementation by reticulocyte haemoglobin content and high-fluorescence reticulocyte count in haemodialysis patients. Nephrol Dial Transplant 18:370–377
Urrechaga E, Boveda O, Aguayo FJ, de la Hera P, Munoz RI, Gallardo I, Escanero JF (2016) Percentage of hypochromic erythrocytes and reticulocyte hemoglobin equivalent predictors of response to intravenous iron in hemodialysis patients. Int J Lab Hematol 38:360–365
Fishbane S, Shapiro W, Dutka P, Valenzuela OF, Faubert J (2001) A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney Int 60:2406–2411
Kaneko Y, Miyazaki S, Hirasawa Y, Gejyo F, Suzuki M (2003) Transferrin saturation versus reticulocyte hemoglobin content for iron deficiency in Japanese hemodialysis patients. Kidney Int 63:1086–1093
Canals C, Remacha AF, Sarda MP, Piazuelo JM, Royo MT, Romero MA (2005) Clinical utility of the new Sysmex XE 2100 parameter-reticulocyte hemoglobin equivalent - in the diagnosis of anemia. Haematologica 90:1133–1134
Lorenz L, Arand J, Buchner K, Wacker-Gussmann A, Peter A, Poets CF, Franz AR (2015) Reticulocyte haemoglobin content as a marker of iron deficiency. Arch Dis Child Fetal Neonatal Ed 100:F198–F202
Parodi E, Giraudo MT, Ricceri F, Aurucci ML, Mazzone R, Ramenghi U (2016) Absolute reticulocyte count and reticulocyte hemoglobin content as predictors of early response to exclusive oral iron in children with iron deficiency anemia. Anemia 2016:7345835
Syed S, Kugathasan S, Kumar A, Prince J, Schoen BT, McCracken C, Ziegler TR, Suchdev PS (2017) Use of reticulocyte hemoglobin content in the assessment of iron deficiency in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 64:713–720
Davidkova S, Prestidge TD, Reed PW, Kara T, Wong W, Prestidge C (2016) Comparison of reticulocyte hemoglobin equivalent with traditional markers of iron and erythropoiesis in pediatric dialysis. Pediatr Nephrol 31:819–826
Lopez-Ruzafa E, Vazquez-Lopez MA, Lendinez-Molinos F, Poveda-Gonzalez J, Galera-Martinez R, Bonillo-Perales A, Martin-Gonzalez M (2016) Reference values of reticulocyte hemoglobin content and their relation with other indicators of Iron status in healthy children. J Pediatr Hematol Oncol 38:e207–e212
Teixeira C, Barbot J, Freitas MI (2015) Reference values for reticulocyte parameters and hypochromic RBC in healthy children. Int J Lab Hematol 37:626–630
Locatelli F, Aljama P, Barany P, Canaud B, Carrera F, Eckardt KU, Horl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S, European Best Practice Guidelines Working G (2004) Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19(Suppl 2):ii1–i47
Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, Gammaro L, Brocco G, Restivo G, Bernich P, Lupo A, Maschio G (2001) The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant 16:1416–1423
Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I, British Committee for Standards in H (2013) Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol 161:639–648
Urrechaga E (2009) Clinical utility of the new Beckman-Coulter parameter red blood cell size factor in the study of erithropoiesis. Int J Lab Hematol 31:623–629
Kohgo Y, Niitsu Y, Kondo H, Kato J, Tsushima N, Sasaki K, Hirayama M, Numata T, Nishisato T, Urushizaki I (1987) Serum transferrin receptor as a new index of erythropoiesis. Blood 70:1955–1958
Fernandez-Rodriguez AM, Guindeo-Casasus MC, Molero-Labarta T, Dominguez-Cabrera C, Hortal-Casc NL, Perez-Borges P, Vega-Diaz N, Saavedra-Santana P, Palop-Cubillo L (1999) Diagnosis of iron deficiency in chronic renal failure. Am J Kidney Dis 34:508–513
Chen YC, Hung SC, Tarng DC (2006) Association between transferrin receptor-ferritin index and conventional measures of iron responsiveness in hemodialysis patients. Am J Kidney Dis 47:1036–1044
Drakou A, Margeli A, Theodorakopoulou S, Agrogiannis I, Poziopoulos C, Papassotiriou I, Vlahakos DV (2016) Assessment of serum bioactive hepcidin-25, soluble transferrin receptor and their ratio in predialysis patients: correlation with the response to intravenous ferric carboxymaltose. Blood Cells Mol Dis 59:100–105
Lamola AA, Eisinger J, Blumberg WE (1980) Erythrocyte protoporphyrin/heme ratio by hematofluorometry. Clin Chem 26:677–678
Garrett S, Worwood M (1994) Zinc protoporphyrin and iron-deficient erythropoiesis. Acta Haematol 91:21–25
Hastka J, Lasserre JJ, Schwarzbeck A, Strauch M, Hehlmann R (1993) Zinc protoporphyrin in anemia of chronic disorders. Blood 81:1200–1204
Fishbane S, Lynn RI (1995) The utility of zinc protoporphyrin for predicting the need for intravenous iron therapy in hemodialysis patients. Am J Kidney Dis 25:426–432
Braun J, Hammerschmidt M, Schreiber M, Heidler R, Horl WH (1996) Is zinc protoporphyrin an indicator of iron-deficient erythropoiesis in maintenance haemodialysis patients? Nephrol Dial Transplant 11:492–497
Moreb J, Popovtzer MM, Friedlaender MM, Konijn AM, Hershko C (1983) Evaluation of iron status in patients on chronic hemodialysis: relative usefulness of bone marrow hemosiderin, serum ferritin, transferrin saturation, mean corpuscular volume and red cell protoporphyrin. Nephron 35:196–200
Wish JB (2006) Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 1(Suppl 1):S4–S8
Ozment CP, Turi JL (2009) Iron overload following red blood cell transfusion and its impact on disease severity. Biochim Biophys Acta 1790:694–701
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Answers
1. e; 2. d; 3. e; 4. a; 5. e
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hayes, W. Measurement of iron status in chronic kidney disease. Pediatr Nephrol 34, 605–613 (2019). https://doi.org/10.1007/s00467-018-3955-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-3955-x