Abstract
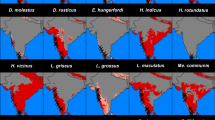
Small ectotherms, such as insects, with high surface area-to-volume ratios are usually at risk of dehydration in arid environments. We hypothesize that desiccation tolerance in insects could be reflected in their distribution, which is limited by areas with high relative values of water vapor pressure deficit (VPD) (e.g., hot and dry). The main goal of this study was to explore whether incorporation of eco-physiological traits such as desiccation tolerance in arid environments can improve our understanding of species distribution models (SDM). We use a novel eco-physiological approach to understand the distribution and the potential overlap with their fundamental niche in triatomine bugs, Chagas disease vectors. The desiccation dimension for T. infestans, T. delpontei, T. dimidiata, and T. sordida niches seems to extend to very dry areas. For T. vitticeps, xeric areas seem to limit the geographical range of their realized niche. The maximum VPD limits the western and southern distributions of T. vitticeps, T. delpontei, and T. patagonica. All species showed high tolerance to desiccation with survival times (35 °C-RH ~ 15%) ranging from 24 to 38 days, except for T. dimidiata (9 days), which can be explained by a higher water-loss rate, due to a higher cuticular permeability along with a higher critical water content. This approach indicates that most of these triatomine bugs could be exploiting the dryness dimension of their fundamental niche. Incorporating such species-specific traits in studies of distribution, range, and limits under scenarios of changing climate could enhance predictions of movement of disease-causing vectors into novel regions.



Similar content being viewed by others
References
Araújo M, Ferri-Yáñez F, Bozinovic F, Marquet P, Valladares F, Chown S (2013) Heat freezes niche evolution. Ecol Lett 16:1206–1219
Balsalobre A (2016) Ph-D Thesis: ¿Qué especies de vinchucas modificarán su distribución geográfica en la Argentina? Un análisis de los microhábitats y microclimas de los triatominos vectores de la enfermedad de Chagas. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Argentina
Belliard S (2015) Degree Thesis. Plasticidad de la tolerancia térmica por aclimatación en la vinchuca Rhodnius prolixus. Universidad de Buenos Aires, Argentina
Benoit J, Denlinger D (2010) Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods. J Insect Physiol 56(10):1366–1376
Buckley L, Urban M, Angilletta M, Crozier L, Rissler L, Sears M (2010) Can mechanism inform species’ distribution models? Ecol Lett 13(8):1041–1054
Bujan J, Yanoviak SP, Kaspari M (2016) Desiccation resistance in tropical insects: causes and mechanisms underlying variability in a Panama ant community. Ecol Evol 6(17):6282–6291
Bulleri F, Bruno JF, Silliman BR, Stachowicz JJ (2016) Facilitation and the niche: implications for coexistence, range shifts and ecosystem functioning. Funct Ecol 30(1):70–78
Carcavallo RU, Curto de Casas SI, Sherlock IA, Galíndez-Girón I, Jurberg J, Galvão C, Noireau F (1999) Geographical distribution and alti-latitudinal dispersion. Atlas Chagas Dis Vect Am 3:747–792
Chown S, Nicolson S (2004) Insect physiological ecology. Oxford University Press, New York, p 244
Chown S, Sørensen J, Terblanche J (2011) Water loss in insects: an environmental change perspective. J Insect Physiol 57(8):1070–1084
Clark N (1935) The effect of temperature and humidity upon the eggs of the bug, Rhodnius prolixus (Heteroptera, Reduviidae). J Anim Ecol 4:82–87
Coast GM (2009) Neuroendocrine control of ionic homeostasis in blood-sucking insects. J Exp Biol 212:378–386
Colwell RK, Rangel TF (2009) Hutchinson’s duality: the once and future niche. Proc Natl Acad Sci USA 106(Suppl. 2):19651–19658
de la Vega GJ, Schilman PE (2017) Ecological and physiological thermal niches in vectors of Chagas disease. Med Vet Entomol. doi:10.1111/mve.12262
de la Vega GJ, Medone P, Ceccarelli S, Rabinovich J, Schilman PE (2015) Geographical distribution, climatic variability and thermo-tolerance of Chagas disease vectors. Ecography 38(8):851–860
de Souza R, Diotaiuti L, Lorenzo M, Gorla DE (2010) Analysis of the geographical distribution of Triatoma vitticeps (Stal, 1859) based on data of species occurrence in Minas Gerais, Brazil. J Infec Genet Evol 10(6):720–760
Denny M (2016) Ecological mechanics. Principles of life’s physical interactions. Princeton University Press, Princeton
Diniz-Filho JAF, Ceccarelli S, Hasperué W, Rabinovich J (2013) Geographical patterns of Triatominae (Heteroptera: Reduviidae) richness and distribution in the Western Hemisphere. Insect Conserv Divers 6:704–714
Edney E (1977) Water balance in land arthropods. Springer, Germany
Elith J, Kearney M, Phillips S (2010) The art of modelling range-shifting species. Methods Ecol Evol 1(4):330–342
Felsenstein J (1985) Phylogenies and comparative method. Am Nat 125(1):1–15
Fergnani P, Ruggiero A, Ceccarelli S, Menu F, Rabinovich J (2013) Large-scale patterns in morphological diversity and species assemblages in Neotropical Triatominae (Heteroptera: Reduviidae). Mem Inst Oswaldo Cruz 108(8):997–1008
Fourcade Y, Engler JO, Rödder D, Secondi J (2014) Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PLoS One 9(5):e97122
Gibbs A (2002) Water balance in desert Drosophila: lessons from non-charismatic. Comp Biochem Physiol Part A 133:781–789
Gouveia S, Hortal J, Tejedo M, Duarte H, Cassemiro F, Navas C, Diniz-filho JAF (2014) Climatic niche at physiological and macroecological scales: the thermal tolerance geographical range interface and niche dimensionality. Glob Ecol Biogeogr 23:446–456
Graham CH, Hijmans RJ (2006) A comparison of methods for mapping species ranges and species richness. Glob Ecol Biogeogr 15(6):578–587
Gurgel-Gonçalves R, Galvao C, Costa J, Peterson AT (2012) Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med 2012:1–15
Hadley NF (1994) Water relations of terrestrial arthropods. Academic Press Inc, San Diego, California, p 356
Hijmans RJ, van Etten J (2015) raster: Geographic data analysis and modeling. R package version 2(1-49):2013
Hijmans RJ, Phillips S, Leathwick J, Elith J (2015) dismo: Species distribution modeling. R package version 1.0-12
Hill M, Hoffmann A, Macfadyen S, Umina P, Elith J (2012) Understanding niche shifts: using current and historical data to model the invasive redlegged earth mite, Halotydeus destructor. Divers Distrib 18(2):191–203
Hutchinson GE (1957) Concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427
Hypsa V, Tietz D, Zrzavý J, Rego R, Galvao C, Jurberg J (2002) Phylogeny and biogeography of Triatominae (Hemiptera: Reduviidae): molecular evidence of a New World origin of the Asiatic clade. Mol Phylogenet Evol 23(3):447–457
Intergovernmental Panel on Climate Change (2014) Impacts, adaptation and vulnerability: regional aspects. Cambridge University Press, New York
Jiménez-Valverde A, Lobo JM (2007) Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecol 31:361–369
Jurenka R, Terblanche JS, Klok CJ, Chown SL, Krafsur ES (2007) Cuticular lipid mass and desiccation rates in Glossina pallidipes: interpopulation variation. Physiol Entomol 32(3):287–293
Kearney M (2006) Habitat, environment and niche: what are we modelling? Oikos 115(1):186–191
Kleynhans E, Terblanche J (2009) The evolution of water balance in Glossina (Diptera: Glossinidae): correlations with climate. Biol Lett 5:93–96
Kleynhans E, Terblanche J (2011) Complex interactions between temperature and relative humidity on water balance of adult tsetse (Glossinidae, Diptera): implications for climate change. Front Physiol 2(74):1–10
Klok J, Chown S (1997) Critical Thermal Limits, Temperature Tolerance and Water Balance of a Sub-Antarctic Caterpillar, Pringleophaga marioni (Lepidoptera: Tineidae). J Insect Physiol 43(7):685–694
Lapinski W, Tschapka M (2014) Desiccation resistance reflects patterns of microhabitat choice in a Central American assemblage of wandering spiders. J Exp Biol 217(15):2789–2795
Lorenzo M, Lazzari CR (1999) Temperature and relative humidity affect the selection of shelters by Triatoma infestans, vector of Chagas disease. Acta Trop 72:241–249
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11(10):995–1003
Luz C, Fargues J, Grunewald J (1999) Development of Rhodnius prolixus (Hemiptera: Reduviidae) under constant and cyclic conditions of temperature and humidity. Mem Inst Oswaldo Cruz 94(3):403–409
Lyons CL, Coetzee M, Terblanche J, Chown S (2012) Thermal limits of wild and laboratory strains of two African malaria vector species, Anopheles arabiensis and Anopheles funestus. Malar J 11:226
Mac Arthur R (1984) Geographical ecology: patterns in the distribution of species. Harper and Row, New York, p 288
Martin P, Lefebvre M (1995) Malaria and climate: sensitivity of potential transmission to climate. Ambio 24(4):200–207
Mitchell T, Carter T, Jones P, Hulme M, New M (2004) A comprehensive set of climate scenarios for Europe and the globe: the observed record (1900–2000) and 16 scenarios (2000–2100). University of East Anglia, Norwich, p 30
Monahan WB (2009) A mechanistic niche model for measuring species’ distributional responses to seasonal temperature gradients. PLoS One 4(11):e7921
Nenzén HK, Araújo MB (2011) Choice of threshold alters projections of species range shifts under climate change. Ecol Modell 222(18):3346–3354
Orme D (2013) The caper package: comparative analysis of phylogenetics and evolution in R. R package version 5(2):1–36
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190(3):231–259
Pinheiro J, Bates D, DebRoy S, Sarkar D (2014) The nlme package: linear and nonlinear mixed effects models. R package version 3:1–131
Pires H, Lazzari CR, Schilman PE, Diotaiuti L, Lorenzo M (2002) Dynamics of thermopreference in the Chagas disease vector Panstrongylus megistus (Hemiptera: Reduviidae). J Med Entomol 39(5):716–719
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Richmond O, McEntee J, Hijmans R, Brashares J (2010) Is the climate right for pleistocene rewilding? Using species distribution models to extrapolate climatic suitability for mammals across continents. PLoS One 5(9):e12899
Roca M, Lazzari CR (1994) Effects of the relative humidity on the haematophagous bug Triatoma infestans. Higropreference and eclosion success. J Insect Physiol 40:901–907
Rolandi C, Schilman PE (2012) Linking global warning, metabolic rate of haematophagous vectors and the transmission of infectious diseases. Front Physiol 3(75):1–3
Rolandi C, Iglesias M, Schilman PE (2014) Metabolism and water loss rate of the haematophagous insect Rhodnius prolixus: effect of starvation and temperature. J. Exp Biol 217:4414–4422
Schilman PE, Lighton JRB, Holway DA (2005) Respiratory and cuticular water loss in insects with continuous respiration: comparison across five different ant species. J Insect Physiol 51(12):1295–1305
Schilman PE, Lighton JRB, Holway D (2007) Water balance in the Argentine ant (Linepithema humile) compared with five common native ant species from southern California. Physiol Entomol 32(1):1–7
Schilman PE, Minoli S, Lazzari CR (2009) The adaptive value of hatching towards the end of the night: lessons from eggs of the haematophagous bug Rhodnius prolixus. Physiol Entomol 34(3):231–237
Svenning J, Normand S, Kageyama M (2008) Glacial refugia of temperate trees in Europe: insights from species distribution modelling. J Ecol 96(6):1117–1127
Tee H, Lee C (2015) Water balance profiles, humidity preference and survival of two sympatric cockroach egg parasitoids Evania appendigaster and prostocetus hagenowii (Hymenoptera: Evaniidae; Eulophidae). J Insect Physiol 77:45–54
Tingley R, Vallinoto M, Sequeira F, Kearney M (2014) Realized niche shift during a global biological invasion. Proc Natl Acad Sci USA 111(28):10233–10238
Weldon CW, Boardman L, Marlin D, Terblanche JS (2016) Physiological mechanisms of dehydration tolerance contribute to the invasion potential of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) relative to its less widely distributed congeners. Front Zool 13:15
WHO Expert Committee World Health Organization (2002) Control of Chagas disease Second report of the WHO. Tech Rep Ser 905:1–119
Wigglesworth VB (1945) Transpiration through the cuticle of insects. J Exp Biol 21(3–4):97–114
Zachariassen K (1996) The water conserving physiological compromise of desert insects. Eur J Entomol 3:359–367
Zuur A, Ieno E, Elphick C (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14
Acknowledgements
The authors thanks to Dr. Brian Aukema and Jake Wittman from the Aukema Lab (http://www.forest-insects.umn.edu) for critical reading of the manuscript, Amir Dyzenchauz for English corrections, Carmen Rolandi for helping with figures, and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina) (PICT2008-0035 and PICT2008-0268) and CONICET for past financial support. We also thank two anonymous reviewers and a handling editor, whose constructive comments improved the paper.
Author information
Authors and Affiliations
Contributions
Conceived the idea and designed the experiments: PES and GJdlV. Experimental assay: GJdlV and PES. Data analysis: GJdlV. Led the writing of the manuscript: GJdlV. Contributed reagents/materials: PES.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Sylvain Pincebourde.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de la Vega, G.J., Schilman, P.E. Using eco-physiological traits to understand the realized niche: the role of desiccation tolerance in Chagas disease vectors. Oecologia 185, 607–618 (2017). https://doi.org/10.1007/s00442-017-3986-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3986-1