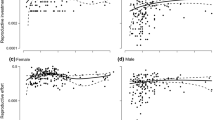
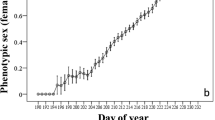
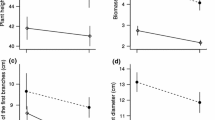
Abstract
Excess flower production is a common phenomenon in hermaphrodite plants. The tropical pioneer shrub Melastoma malabathricum (Melastomataceae) frequently aborts not only young ovaries just after flowering, but also flower buds and developed ovaries. We tested a hypothesis that the excess production of reproductive organs and their abortion in this species is an adaptation to environmental fluctuations over shorter time scales than had previously been reported in other plants. To calculate the daily demand for carbohydrate and water by reproductive organs at the level of individual plants, we measured the respiration and transpiration of the reproductive organs at various stages and monitored their growth and abortion. To determine the daily supply of carbohydrate and water, we measured the photosynthetic productivity of leaf area, solar radiation and rainfall. The daily carbohydrate demands of the reproductive organs were significantly correlated with total photosynthetic productivity per leaf area during the previous 1, 3 and 5 days, but no correlations were found between the demands for water and accumulated rainfall or radiation. The daily abortion rates of the population were also correlated with demand for carbohydrates on the previous day per total photosynthetic productivity per leaf area. In brief, it was considered that this species produced and grew more reproductive organs when more resources were supplied and that the abortion occurred when demands for carbohydrate were large. Therefore our hypothesis was supported. We concluded that this reproductive strategy was an adaptation for pioneers characterized by continuous reproduction in aseasonal tropics. In our study, the adaptive consequence of excess production was determined by measuring natural environmental fluctuation.







Similar content being viewed by others
References
Amthor JS (1989) Respiration and crop productivity. Springer, New York
Bernasconi G (2003) Seed paternity in flowering plants: an evolutionary perspective. Perspect Plant Ecol Evol Syst 6:149–158
Boote KJ, Loomis RS (1991) The prediction of canopy assimilation. In: Boote KJ, Loomis RS (eds) Modeling crop photosynthesis—from biochemistry to canopy. Crop Science Society of America, Madison, Wis., pp 109–140
Burd M (1998) “Excess” flower production and selective fruit abortion: a model of potential benefits. Ecology 79:2123–2132
Burd M, Challahan HS (2000) What does the male function hypothesis claim? J Evol Biol 13:735–742
Burd M (2004) Offspring quality in relation to excess flowers in Pultenaea gunnii (Fabaceae). Evolution 58:2371–2376
Chapin FS III, Schulze ED, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Clark DA, Clark DB (1992) Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecol Monogr 62:315–344
Corner EJH (1988) Wayside trees of Malaya, 3rd edn. The Malayan Nature Society, Kuala Lumpur
Coulter MC (1979) Seed abortion in Anagallis arvensis on southeast Farallon Island, California. Madroño 26:101–102
Cowan AA, Marshall AH, Michaelson-Yeates TPT (2000) Effect of pollen competition and stigmatic receptivity on seed set in white clover (Trifolium repens L.). Sex Plant Reprod 13:37–42
Delph LF, Weinig C, Sullivan K (1998) Why fast-growing pollen tubes give rise to vigorous progeny: the test of a new mechanism. Proc R Soc Lond B 265:935–939
Eriksson O (1987) Regulation of seed-set and gender variation in the hermaphroditic plant Potentilla anserine. Oikos 49:165–171
Fretwell SD, Lucas HL (1969) On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheoret 19:16–36
Gamalei YV (2002) Assimilate transport and partitioning in plants: approaches, methods, and facets of research. Russ J Plant Physiol 49:16–31
Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156:145–169
Gorchov DL (1988) Effects of pollen and resources on seed number and other fitness components in Amelanchier arborea (Rosaceae: Maloideae). Am J Bot 75:1275–1285
Holtsford TP (1985) Nonfruiting hermaphroditic flowers of Calochortus leichtlinii (Liliaceae): potential reproductive functions. Am J Bot 72:1687–1694
Huth CJ, Pellmyr O (2000) Pollen-mediated selective abortion in yuccas and its consequences for the plant–pollinator mutualism. Ecology 81:1100–1107
Ichie T, Kenta T, Nakagawa M, Sato K, Nakashizuka T (2005a) Resource allocation to reproductive organs during masting in the tropical emergent tree, Dipterocarpus tempehes. J Trop Ecol 21:237–241
Ichie T, Kenzo T, Kitahashi Y, Koike T, Nakashizuka T (2005b) How does Dryobalanops aromatica supply carbohydrate resources for reproduction in a masting year? Trees 19:703–710
Kato M., Inoue T, Hamid AA, Nagamitsu T, Merdek MB, Nona AR, Itino T, Yamane S, Yumoto T (1995) Seasonality and vertical structure of light-attracted insect communities in a dipterocarp forest in Sarawak. Res Popul Ecol 37:59–79
Kenzo T, Ichie T, Ninomiya I, Koike T (2003) Photosynthetic activity in seed wings of Dipterocarpaceae in a masting year: does wing photosynthesis contribute to reproduction? Photosynthetica 41:551–557
Korbecka G, Klinkhamer PGL, Vrieling K (2002) Selective embryo abortion hypothesis revisited—a molecular approach. Plant Biol 4:298–310
Körner C (2003) Carbon limitation in trees. J Ecol 91:4–17
Kumagai T, Saitoh TM, Sato Y, Takahashi H, Manfroi OJ, Morooka T, Kuraji K, Suzuki M, Yasunari T, Komatsu H (2005) Annual water balance and seasonality of evapotranspiration in a Bornean tropical rainforest. Agric For Meteorol 128:81–92
Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26:37–56
Lee TD, Bazzaz FA (1982) Regulation of fruit and seed production in an annual legume, Cassia fasciculata. Ecology 63:1363–1373
Meir P, Grace J (2002) Scaling relationships for woody tissue respiration in two tropical rain forests. Plant Cell Environ 25:963–973
Meletiou-Christou MS, Banilas GP, Diamantoglou S (1998) Seasonal trends in energy contents and storage substances of the Mediterranean species Dittrichia viscosa and Thymelaea tartonraira. Environ Exp Bot 39:21–32
Melser C, Rademaker MCJ, Klinkhamer PGL (1997) Selection on pollen donors by Echium vulgare (Boraginaceae). Sex Plant Reprod 10:305–312
Melser C, Klinkhamer PGL (2001) Selective seed abortion increases offspring survival in Cynoglossum officinale (Boraginaceae). Am J Bot 88:1033–1040
Momose K, Ishii R, Sakai S, Inoue T (1998a) Plant reproductive intervals and pollinators in the aseasonal tropics: a new model. Proc R Soc Lond B 265:2333–2339
Momose K, Yumoto T, Nagamitsu T, Kato M, Nagamasu H, Sakai S, Harrison RD, Itioka T, Hamid AA, Inoue T (1998b) Pollination biology in a lowland dipterocarp forest in Sarawak Malaysia. I. Characteristics of the plant–pollinator community in a lowland dipterocarp forest. Am J Bot 85:1477–1501
Montalvo AM (1992) Relative success of self and outcross pollen comparing mixed- and single-donor pollinations in Aquilegia caerulea. Evolution 46:1181–1198
Mooney HA (1972) The carbon balance of plants. Annu Rev Ecol Syst 3:315–346
Nishikawa Y (1998) The function of multiple flowers of a spring ephemeral, Gagea lutea (Liliaceae), with reference to blooming order. Can J Bot 76:1404–1411
Obeso JR (1993) Selective fruit and seed maturation in Asphodelus albus Miller (Liliaceae). Oecologia 93:564–570
Parra-Tabla V, Bullock SH (1998) Factors limiting fecundity of the tropical tree Ipomoea wolcottiana (Convolvulaceae) in a Mexican tropical dry forest. J Trop Ecol 14:615–627
Raspé O, Guillaume P, Jacquemart AL (2004) Inbreeding depression and biased paternity after mixed-pollination in Vaccinium myrtillus L. (Ericaceae). Int J Plant Sci 165:765–771
Sakai S, Momose K, Yumoto T, Nagamitsu T, Nagamasu H, Hamid AA, Nakashizuka T (1999) Plant reproductive phenology over four years including an episode of general flowering in a lowland dipterocarp forest,Sarawak, Malaysia. Am J Bot 86:1414–1436
Sakai S (2000) Reproductive phenology of gingers in a lowland mixed dipterocarp forest in Borneo. J Trop Ecol 16:337–354
Shi XJ, Michaels HJ, Mitchell RJ (2005) Effects of self-pollination and maternal resources on reproduction and offspring performance in the wild lupine, Lupinus perennis (Fabaceae). Sex Plant Reprod 18:55–64
Shimano K (2000) A power function for forest structure and regeneration pattern of pioneer and climax species in patch mosaic forests. Plant Ecol 146:207–220
Stephenson AG, Winsor JA (1986) Lotus corniculatus regulates offspring quality through selective fruit abortion. Evolution 40:453–458
Sutcliffe J (1977) Plant and temperature. Arnold, London
Sutherland S (1986) Patterns of fruit-set: what controls fruit–flower ratios in plants? Evolution 40:117–128
Tjoelker MG, Oleksyn J, Reich PB (2001) Modelling respiration of vegetation: evidence for a general temperature-dependent Q 10. Global Change Biol 7:223–230
Vaughton G (1991) Variation between years in pollen and nutrient limitation of fruit-set in Banksia spinulosa. J Ecol 78:389–400
Willson MF, Price PW (1980) Resource limitation of fruit and seed production in some Asclepias species. Can J Bot 58:2229–2233
Yoneda R, Ninomiya I, Patanaponpaiboon P, Ogino K (2002) Carbohydrate dynamics on trees in dry evergreen forest, Thailand. I. Diel fluctuation of carbohydrates in a whole tree of Hydnocarpus ilicifolius. Tropics 11:125–134
Acknowledgements
The authors thank Tomoaki Ichie, Kochi University, for his support; Shoko Sakai, Kyoto University, for valuable advice; Megumi Yamashita, Kinki Surveyor School, for permission to use unpublished meteorological information; Kyoko Hamamoto and Kazuki Awazawa, Ehime University, for field assistance; Takayoshi Koike, Hokkaido University, for loaning us a conifer chamber; Tomonori Kume, University of Tokyo, for help with meteorological studies; and two anonymous reviewers and the handling editor for valuable comments on this paper. This study was carried out as a part of project no. 2 of the Research Institute of Humanity and Nature organized by Tohru Nakashizuka, and the CREST project organized by Masakazu Suzuki, University of Tokyo. We thank the organizers of these projects and their Malaysian counterparts, Lucy Chong, Sarawak Forest Corporation, and Josef J. Kendawang, Sarawak Forestry Department, as well as other project members. The experiments comply with the current law of Malaysia in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marylin Ball.
Rights and permissions
About this article
Cite this article
Kamoi, T., Kenzo, T., Kuraji, K. et al. Abortion of reproductive organs as an adaptation to fluctuating daily carbohydrate production. Oecologia 154, 663–677 (2008). https://doi.org/10.1007/s00442-007-0864-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0864-2