Abstract
Background
Transthyretin (TTR) is considered to be associated with insulin resistance in humans. This study aimed to investigate TTR level in gestational diabetes mellitus (GDM) and its association with glucose metabolism.
Methods
Fifty pregnant women with GDM and 47 pregnant women with normal glucose tolerance matched for body mass index and age were enrolled in this study. Their blood samples were collected to detect TTR, retinol-binding protein 4 (RBP4), and their association with glucose and lipid metabolism.
Results
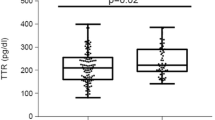
Serum TTR levels in the GDM group were significantly higher than those in the control group (median, 93.44 [interquartile range, 73.81, 117.79] μg/ml vs. 80.83 [74.19, 89.38] μg/ml; P = 0.006). GDM subjects had a lower RBP4/TTR ratio than the control subjects (median, 517.57 [interquartile range, 348.38, 685.27] vs. 602.56 [460.28, 730.62]; P = 0.02). The serum TTR concentrations were positively associated with neonatal weight (r = 0.223, P = 0.028), homeostatic model assessment of insulin resistance (r = 0.246, P = 0.015), and fasting blood glucose (FBG) (r = 0.363, P < 0.001). In stepwise multivariate linear regression analysis, FBG (standardized beta = 0.27, P = 0.004) and neonatal weight (standardized beta = 0.345, P < 0.001) were independent predictors of serum TTR concentrations. Additionally, FBG (standardized beta = − 0.306, P = 0.002) and triglyceride (TG) (beta = 0.219, P = 0.025) were independently associated with RBP4/TTR ratio.
Conclusions
Serum TTR concentrations were significantly higher in women with GDM than that in women without GDM, suggesting that elevated TTR level may play a role in the pathogenesis of GDM. Meanwhile, TTR was positively and independently associated with FBG and neonatal weight, while FBG and TG were independent predictors of RBP4/TTR ratio. Moreover, serum TTR levels and RBP4/TTR ratio were considered valuable markers of insulin resistance and GDM.

Similar content being viewed by others
References
Crowther CA, Hiller JE, Moss JR et al (2011) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 60:2477–2486. https://doi.org/10.1056/NEJMoa042973
Veeraswamy S, Vijayam B, Gupta VK et al (2012) Gestational diabetes: the public health relevance and approach. Diabetes Res Clin Pract 97:350–358. https://doi.org/10.1016/j.diabres.2012.04.024
Choi SH, Kwak SH, Youn BS et al (2008) High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab 93:3142–3148. https://doi.org/10.1210/jc.2007-1755
Dahlgren J (2006) Pregnancy and insulin resistance. Metab Syndr Relat Disord 4:149–152. https://doi.org/10.1089/met.2006.4.149
Blake CCF, Geisow MJ, Oatley SJ et al (1978) Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol 121:339–356. https://doi.org/10.1016/0022-2836(78)90368-6
Ingenbleek Y, Young V (1994) Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr 14:495–533. https://doi.org/10.1146/annurev.nu.14.070194.002431
McKinnon B, Li H, Richard K, Mortimer R (2005) Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J Clin Endocrinol Metab 90:6714–6720. https://doi.org/10.1210/jc.2005-0696
Patel J, Landers KA, Li H, Mortimer RH, Richard K (2011) Ontogenic changes in placental transthyretin. Placenta 32:817–822. https://doi.org/10.1016/j.placenta.2011.09.007
Landers KA, Mortimer RH, Richard K (2013) Transthyretin and the human placenta. Placenta 34:513–517. https://doi.org/10.1016/j.placenta.2013.04.013
Xiong T, Zhong C, Zhou X et al (2017) Maternal circulating transthyretin level is longitudinally associated with increased risk of gestational diabetes mellitus: it is not just an indicator of nutritional status. Diabetes Care 40:e53–e54. https://doi.org/10.2337/dc16-2731
Fasshauer M, Blüher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36:461–470. https://doi.org/10.1016/j.tips.2015.04.014
van Bennekum AM, Wei S et al (2001) Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem 276:1107–1113. https://doi.org/10.1074/jbc.M008091200
Zemany L, Bhanot S, Peroni OD et al (2015) Transthyretin antisense oligonucleotides lower circulating RBP4 levels and improve insulin sensitivity in obesemice. Diabetes 64:1603–1614. https://doi.org/10.2337/db14-0970
Fruscalzo A, Londero AP et al (2015) First trimester concentrations of the ttr-rbp4-retinol complex components as early markers of insulin-treated gestational diabetes mellitus. Clin Chem Lab Med 53:1643–1651. https://doi.org/10.1515/cclm-2014-0929
Thomas MR, Massoudi M, Byrne J et al (1988) Evaluation of transthyretin as a monitor of protein-energy intake in preterm and sick neonatal infants. JPEN J Parenter Enteral Nutr 12:162–166. https://doi.org/10.1177/0148607188012002162
Berry DC, Croniger CM, Ghijselinck NB, Noy A (2012) Transthyretin blocks retinol uptake and cell signaling by the holo-retinol binding protein receptor STRA 6. Mol Cell Biol 32:3851–3859. https://doi.org/10.1128/MCB.00775-12
Kobbah AM, Hellsing K, Tuvemo T (1988) Early changes of some serum proteins and metals in diabetic children. Acta Paediatr Scand 77:734–740. https://doi.org/10.1111/j.1651-2227.1988.tb10739.x
Klöting N, Graham TE, Berndt J et al (2007) Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 6:79–87. https://doi.org/10.1016/j.cmet.2007.06.002
Mody N, Graham TE, Tsuji Y, Yang Q, Kahn BB (2008) Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am J Physiol Endocrinol Metab 294:E785–E793. https://doi.org/10.1152/ajpendo.00521.2007
Su Y, Jono H, Misumi Y et al (2012) Novel function of transthyretin in pancreatic alpha cells. FEBS Lett 586:4215–4222. https://doi.org/10.1016/j.febslet.2012.10.025
Refai E, Dekki N, Yang SN et al (2005) Transthyretin constitutes a functional component in pancreatic beta-cell stimulus-secretion coupling. Proc Natl Acad Sci 102:17020–17025. https://doi.org/10.1073/pnas.0503219102
Liz MA, Gomes CM, Saraiva MJ, Sousa MM (2007) ApoA-I cleaved by transthyretin has reduced ability to promote cholesterol efflux and increased amyloidogenicity. J Lipid Res 48:2385–2395. https://doi.org/10.1194/jlr.M700158-JLR200
Fruscalzo A, Frommer J, Londero AP et al (2017) First trimester TTR-RBP4-ROH complex and angiogenic factors in the prediction of small for gestational age infant’s outcome. Arch Gynecol Obstet 295:1157–1165. https://doi.org/10.1007/s00404-017-4338-4
Krzyzanowska K, Zemany L, Krugluger W et al (2008) Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia 51:1115–1122. https://doi.org/10.1007/s00125-008-1009-9
Scantlebury T, Maslowska M, Cianflone K (1998) Chylomicron-specific enhancement of acylation stimulating protein and precursor protein c3 production in differentiated human adipocytes. J Biol Chem 33:20903–20909. https://doi.org/10.1074/jbc.273.33.20903
Diamanti-Kandarakis E, Livadas S, Kandarakis SA et al (2008) Low free plasma levels of retinol-binding protein 4 in insulin-resistant subjects with polycystic ovary syndrome. J Endocrinol Invest 31:950–955. https://doi.org/10.1007/BF03345631
Tepper BJ, Kim YK, Shete V et al (2010) Serum retinol-binding protein 4 (RBP4) and retinol in a cohort of borderline obese women with and without gestational diabetes. Clin Biochem 43:320–323. https://doi.org/10.1016/j.clinbiochem.2009.10.013
Yoshida A, Matsutani Y, Fukuchi Y, Saito K, Naito M (2006) Analysis of the factors contributing to serum retinol binding protein and transthyretin levels in Japanese adults. J Atheroscler Thromb 13:209–215. https://doi.org/10.5551/jat.13.209
Du M, Wang B, Liang Z et al (2016) The relationship between retinol-binding protein 4 concentrations and gestational diabetes mellitus in Chinese women. Gynecol Obstet Invest 81:174–180. https://doi.org/10.1159/000398794
Zhaoxia L, Mengkai D, Qin F et al (2014) Significance of RBP4 in patients with gestational diabetes mellitus: a case-control study of Han Chinese women. Gynecol Endocrinol 30:161–164. https://doi.org/10.3109/09513590.2013.871515
Acknowledgements
We specifically thank Qi Cheng and Jiansheng Ji at Women’s Hospital School of Medicine Zhejiang University for their support in this study. The authors declared no potential conflicts of the research, authorship, or publication of this article.
Funding
This study was supported by the National Natural Science Foundation of China (8187060362).
Author information
Authors and Affiliations
Contributions
MTL data curation, methodology support, and writing of the manuscript. YMC acquisition of data, study conception, analysis support, project supervision and critical editing of the manuscript. DQC study conception, funding acquisition, methodology, project supervision and critical editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This study was approved by the Institutional Ethics Committee at Women’s Hospital School of Medicine Zhejiang University. The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, M., Chen, Y. & Chen, D. Association between transthyretin concentrations and gestational diabetes mellitus in Chinese women. Arch Gynecol Obstet 302, 329–335 (2020). https://doi.org/10.1007/s00404-020-05599-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05599-y