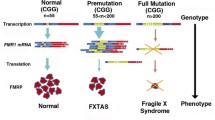
Abstract
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder and is characterized by the progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) and the gradual appearance of α-synuclein (α-syn)-containing neuronal protein aggregates. Although the exact mechanism of α-syn-mediated cell death remains elusive, recent research suggests that α-syn-induced alterations in neuronal excitability contribute to cell death in PD. Because the fragile X mental retardation protein (FMRP) controls the expression and function of numerous neuronal genes related to neuronal excitability and synaptic function, we here investigated the role of FMRP in α-syn-associated pathological changes in cell culture and mouse models of PD as well as in post-mortem human brain tissue from PD patients. We found FMRP to be decreased in cultured DA neurons and in the mouse brain in response to α-syn overexpression. FMRP was, furthermore, lost in the SNc of PD patients and in patients with early stages of incidental Lewy body disease (iLBD). Unlike fragile X syndrome (FXS), FMR1 expression in response to α-syn was regulated by a mechanism involving Protein Kinase C (PKC) and cAMP response element-binding protein (CREB). Reminiscent of FXS neurons, α-syn-overexpressing cells exhibited an increase in membrane N-type calcium channels, increased phosphorylation of ERK1/2, eIF4E and S6, increased overall protein synthesis, and increased expression of Matrix Metalloproteinase 9 (MMP9). FMRP affected neuronal function in a PD animal model, because FMRP-KO mice were resistant to the effect of α-syn on striatal dopamine release. In summary, our results thus reveal a new role of FMRP in PD and support the examination of FMRP-regulated genes in PD disease progression.










Similar content being viewed by others
References
Alisch RS, Wang T, Chopra P, Visootsak J, Conneely KN, Warren ST (2013) Genome-wide analysis validates aberrant methylation in fragile × syndrome is specific to the FMR1 locus. BMC Med Genet 14:1. https://doi.org/10.1186/1471-2350-14-18
Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA (2014) Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30:1363–1369. https://doi.org/10.1093/bioinformatics/btu049
Ashley CT, Wilkinson KD, Reines D, Warren ST (1993) FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262:563–566
Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, Goetz CG, Halliday GM, Hardy J, Lang AE, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2014) Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 29:454–462. https://doi.org/10.1002/mds.25844
Bhakar AL, Dölen G, Bear MF (2012) The Pathophysiology of Fragile × (and what it teaches us about synapses). Annu Rev Neurosci 35:417–443. https://doi.org/10.1146/annurev-neuro-060909-153138
Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E (2012) Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile × syndrome mice. Neuron 76:325–337. https://doi.org/10.1016/j.neuron.2012.07.022
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295. https://doi.org/10.1111/j.1365-2990.2006.00727.x
Bohnen NI, Albin RL (2011) The cholinergic system and Parkinson disease. Behav Brain Res 221:564–573. https://doi.org/10.1016/j.bbr.2009.12.048
Boivin M, Willemsen R, Hukema RK, Sellier C (2017) Potential pathogenic mechanisms underlying Fragile × Tremor Ataxia Syndrome: RAN translation and/or RNA gain-of-function? Eur J Med Genet. https://doi.org/10.1016/j.ejmg.2017.11.001
Bolam JP, Pissadaki EK (2012) Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord 27:1478–1483. https://doi.org/10.1002/mds.25135
Braak H, Braak H, Del Tredici K, Del Tredici K (2009) Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol 201:1–119
Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Brager DH, Akhavan AR, Johnston D (2012) Impaired dendritic expression and plasticity of h-channels in the fmr1(−/y) mouse model of fragile × syndrome. Cell Rep 1:225–233. https://doi.org/10.1016/j.celrep.2012.02.002
Brichta L, Greengard P (2014) Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Front Neuroanat 8:152. https://doi.org/10.3389/fnana.2014.00152
Brown MR, Kronengold J, Gazula V-R, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK (2010) Fragile × mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci 13:819–821. https://doi.org/10.1038/nn.2563
Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST (2001) Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile × syndrome. Cell 107:477–487
Bruch J, Xu H, De Andrade A, Höglinger G (2014) Mitochondrial complex 1 inhibition increases 4-repeat isoform tau by SRSF2 upregulation. PLoS One 9:e113070. https://doi.org/10.1371/journal.pone.0113070
Bruch J, Xu H, Rösler TW, De Andrade A, Kuhn P-H, Lichtenthaler SF, Arzberger T, Winklhofer KF, Müller U, Höglinger GU (2017) PERK activation mitigates tau pathology in vitro and in vivo. EMBO Mol Med 9:371–384. https://doi.org/10.15252/emmm.201606664
Carbone C, Costa A, Provensi G, Mannaioni G, Masi A (2017) The hyperpolarization-activated current determines synaptic excitability, calcium activity and specific viability of substantia nigra dopaminergic neurons. Front Cell Neurosci 11:251-14. https://doi.org/10.3389/fncel.2017.00187
Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST (2003) Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet 12:3295–3305. https://doi.org/10.1093/hmg/ddg350
Ceravolo R, Antonini A, Volterrani D, Rossi C, Goldwurm S, Di Maria E, Kiferle L, Bonuccelli U, Murri L (2005) Dopamine transporter imaging study in parkinsonism occurring in fragile × premutation carriers. Neurology 65:1971–1973. https://doi.org/10.1212/01.wnl.0000188821.51055.52
Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ (2007) “Rejuvenation” protects neurons in mouse models of Parkinson’s disease. Nature 447:1081–1086. https://doi.org/10.1038/nature05865
Chartier-Harlin M-C, Dachsel JC, Vilariño-Güell C, Lincoln SJ, Leprêtre F, Hulihan MM, Kachergus J, Milnerwood AJ, Tapia L, Song M-S, Le Rhun E, Mutez E, Larvor L, Duflot A, Vanbesien-Mailliot C, Kreisler A, Ross OA, Nishioka K, Soto-Ortolaza AI, Cobb SA, Melrose HL, Behrouz B, Keeling BH, Bacon JA, Hentati E, Williams L, Yanagiya A, Sonenberg N, Lockhart PJ, Zubair AC, Uitti RJ, Aasly JO, Krygowska-Wajs A, Opala G, Wszolek ZK, Frigerio R, Maraganore DM, Gosal D, Lynch T, Hutchinson M, Bentivoglio AR, Valente EM, Nichols WC, Pankratz N, Foroud T, Gibson RA, Hentati F, Dickson DW, Destée A, Farrer MJ (2011) Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet 89:398–406. https://doi.org/10.1016/j.ajhg.2011.08.009
Chartier-Harlin MC, Chartier-Harlin M-C, Kachergus J, Kachergus J, Roumier C, Roumier C, Mouroux V, Mouroux V, Douay X, Douay X, Lincoln S, Lincoln S, Levecque C, Levecque C, Larvor L, Larvor L, Andrieux J, Andrieux J, Hulihan M, Hulihan M, Waucquier N, Waucquier N, Defebvre L, Defebvre L, Amouyel P, Amouyel P, Farrer M, Farrer M, Destee A, Destée A (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169. https://doi.org/10.1016/S0140-6736(04)17103-1
Chen Y-C, Wu Y-R, Mesri M, Chen C-M (2016) Associations of matrix metalloproteinase-9 and tissue inhibitory factor-1 polymorphisms with Parkinson disease in Taiwan. Medicine (Baltimore) 95:e2672. https://doi.org/10.1097/MD.0000000000002672
Cilia R, Kraff J, Canesi M, Pezzoli G, Goldwurm S, Amiri K, Tang H-T, Pan R, Hagerman PJ, Tassone F (2009) Screening for the presence of FMR1 premutation alleles in women with parkinsonism. Arch Neurol 66:244–249. https://doi.org/10.1001/archneurol.2008.548
Contractor A, Klyachko VA, Portera-Cailliau C (2015) Altered neuronal and circuit excitability in fragile × syndrome. Neuron 87:699–715. https://doi.org/10.1016/j.neuron.2015.06.017
Costa A, Gao L, Carrillo F, Cáceres-Redondo MT, Carballo M, Díaz-Martín J, Gómez-Garre P, Sobrino F, Lucas M, López-Barneo J, Mir P, Pintado E (2011) Intermediate alleles at the FRAXA and FRAXE loci in Parkinson’s disease. Parkinsonism Relat Disord 17:281–284. https://doi.org/10.1016/j.parkreldis.2010.12.013
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8):1437–1448
Danesi C, Achuta VS, Corcoran P, Peteri U-K, Turconi G, Matsui N, Albayrak I, Rezov V, Isaksson A, Castrén ML (2018) Increased calcium influx through L-type calcium channels in human and mouse neural progenitors lacking fragile × mental retardation protein. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2018.11.003
Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146:247–261. https://doi.org/10.1016/j.cell.2011.06.013
De Pablo-Fernandez E, Doherty KM, Holton JL, Revesz T, Djamshidian A, Limousin P, Bhatia KP, Warner TT, Lees AJ, Ling H (2015) Concomitant fragile X-associated tremor ataxia syndrome and Parkinson’s disease: a clinicopathological report of two cases. J Neurol Neurosurg Psychiatr 86:934–936. https://doi.org/10.1136/jnnp-2014-309460
Del Tredici K, Braak H (2016) Review: sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol 42:33–50. https://doi.org/10.1111/nan.12298
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080. https://doi.org/10.1001/archneur.65.8.1074
Deng P-Y, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA (2013) FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77:696–711. https://doi.org/10.1016/j.neuron.2012.12.018
Dijkstra AA, Voorn P, Berendse HW, Groenewegen HJ, Bank Netherlands Brain, Rozemuller AJM, van de Berg WDJ (2014) Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov Disord 29:1244–1251. https://doi.org/10.1002/mds.25952
Dölen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF (2007) Correction of fragile × syndrome in mice. Neuron 56:955–962. https://doi.org/10.1016/j.neuron.2007.12.001
Dragicevic E, Schiemann J, Liss B (2015) Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 284:798–814. https://doi.org/10.1016/j.neuroscience.2014.10.037
Dury AY, El Fatimy R, Tremblay S, Rose TM, Côté J, De Koninck P, Khandjian EW (2013) Nuclear fragile × mental retardation protein is localized to cajal bodies. PLoS Genet 9:e1003890. https://doi.org/10.1371/journal.pgen.1003890
Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM (1997) Fragile × mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci 17:1539–1547
Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC (2014) Fragile × mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun 5:1–14. https://doi.org/10.1038/ncomms4628
Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC (2014) Fragile × mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun 5:3628. https://doi.org/10.1038/ncomms4628
Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Warren ST (1991) Variation of the CGG repeat at the fragile × site results in genetic instability: resolution of the Sherman paradox. Cell 67:1047–1058
Fujioka S, Sundal C, Strongosky AJ, Castanedes MC, Rademakers R, Ross OA, Vilariño-Güell C, Farrer MJ, Wszolek ZK, Dickson DW (2013) Sequence variants in eukaryotic translation initiation factor 4-gamma (eIF4G1) are associated with Lewy body dementia. Acta Neuropathol 125:425–438. https://doi.org/10.1007/s00401-012-1059-4
Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M, Gaboury LA, Pandolfi PP, Saad F, Sonenberg N (2010) eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA 107:14134–14139. https://doi.org/10.1073/pnas.1005320107
Fussi N, Höllerhage M, Chakroun T, Nykänen N-P, Rösler TW, Koeglsperger T, Wurst W, Behrends C, Höglinger GU (2018) Exosomal secretion of α-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis 9:757. https://doi.org/10.1038/s41419-018-0816-2
Gehrke S, Wu Z, Klinkenberg M, Sun Y, Auburger G, Guo S, Lu B (2015) PINK1 and Parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metab 21:95–108. https://doi.org/10.1016/j.cmet.2014.12.007
Giguère N, Burke Nanni S, Trudeau L-É (2018) On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol 9:455. https://doi.org/10.3389/fneur.2018.00455
Gkogkas CG, Khoutorsky A, Cao R, Jafarnejad SM, Prager-Khoutorsky M, Giannakas N, Kaminari A, Fragkouli A, Nader K, Price TJ, Konicek BW, Graff JR, Tzinia AK, Lacaille J-C, Sonenberg N (2014) Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile × syndrome-like phenotypes. Cell Rep 9:1742–1755. https://doi.org/10.1016/j.celrep.2014.10.064
Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, Vasuta C, Yee S, Truitt M, Dallaire P, Major F, Lasko P, Ruggero D, Nader K, Lacaille J-C, Sonenberg N (2013) Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 493:371–377. https://doi.org/10.1038/nature11628
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9:13–24. https://doi.org/10.1038/nrneurol.2012.242
Greene JG, Dingledine R, Greenamyre JT (2005) Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis 18:19–31. https://doi.org/10.1016/j.nbd.2004.10.003
Grigsby J (2016) The fragile × mental retardation 1 gene (FMR1): historical perspective, phenotypes, mechanism, pathology, and epidemiology. Clin Neuropsychol 30:815–833. https://doi.org/10.1080/13854046.2016.1184652
Grimm J, Mueller A, Hefti F, Rosenthal A (2004) Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci USA 101:13891–13896. https://doi.org/10.1073/pnas.0405340101
Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ (2011) Fragile × mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci 31:5693–5698. https://doi.org/10.1523/JNEUROSCI.6661-10.2011
Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, Kondapalli J, Schumacker PT, Surmeier DJ (2018) Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J Clin Invest 128:2266–2280. https://doi.org/10.1172/JCI95898
Guzman JN, Sanchez-Padilla J, Chan CS, Surmeier DJ (2009) Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci 29:11011–11019. https://doi.org/10.1523/JNEUROSCI.2519-09.2009
Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468:696–700. https://doi.org/10.1038/nature09536
Hagerman PJ, Hagerman RJ (2015) Fragile X-associated tremor/ataxia syndrome. Ann N Y Acad Sci 1338:58–70. https://doi.org/10.1111/nyas.12693
Hagerman RJ, Hagerman P (2016) Fragile X-associated tremor/ataxia syndrome—features, mechanisms and management. Nat Rev Neurol 12:403–412. https://doi.org/10.1038/nrneurol.2016.82
Hall DA, Berry-Kravis E, Jacquemont S, Rice CD, Cogswell J, Zhang L, Hagerman RJ, Hagerman PJ, Leehey MA (2005) Initial diagnoses given to persons with the fragile × associated tremor/ataxia syndrome (FXTAS). Neurology 65:299–301. https://doi.org/10.1212/01.wnl.0000168900.86323.9c
Hall DA, Howard K, Hagerman R, Leehey MA (2009) Parkinsonism in FMR1 premutation carriers may be indistinguishable from Parkinson disease. Parkinsonism Relat Disord 15:156–159. https://doi.org/10.1016/j.parkreldis.2008.04.037
Hall DA, Jennings D, Seibyl J, Tassone F, Marek K (2010) FMR1 gene expansion and scans without evidence of dopaminergic deficits in parkinsonism patients. Parkinsonism Relat Disord 16:608–611. https://doi.org/10.1016/j.parkreldis.2010.07.006
Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB (1990) Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107. https://doi.org/10.1016/0006-8993(90)90733-r
Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB (1990) Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 27:373–385. https://doi.org/10.1002/ana.410270405
Halliday GM, McRitchie DA, Cartwright H, Pamphlett R, Hely MA, Morris JG (1996) Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J Clin Neurosci 3:52–60
Healy DG, Bressman S, Dickson J, Silveira-Moriyama L, Schneider SA, Sullivan SSO, Massey L, Shaw K, Bhatia KP, Bomanji J, Wood NW, Lees AJ (2009) Evidence for pre and postsynaptic nigrostriatal dysfunction in the fragile × tremor-ataxia syndrome. Mov Disord 24:1245–1247. https://doi.org/10.1002/mds.22267
Hollerhage M, Goebel JN, de Andrade A, Hildebrandt T, Dolga A, Culmsee C, Oertel WH, Hengerer B, Hoglinger GU (2014) Trifluoperazine rescues human dopaminergic cells from wild-type alpha-synuclein-induced toxicity. Neurobiol Aging 35:1700–1711. https://doi.org/10.1016/j.neurobiolaging.2014.01.027
Hoozemans JJM, van Haastert ES, Eikelenboom P, de Vos RAI, Rozemuller JM, Scheper W (2007) Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun 354:707–711. https://doi.org/10.1016/j.bbrc.2007.01.043
Hornykiewicz O (2002) Dopamine miracle: from brain homogenate to dopamine replacement. Mov Disord 17:501–508. https://doi.org/10.1002/mds.10115
Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E (2006) Dynamic translational and proteasomal regulation of fragile × mental retardation protein controls mGluR-dependent long-term depression. Neuron 51:441–454. https://doi.org/10.1016/j.neuron.2006.07.005
Huntley GW (2012) Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci 13:743–757. https://doi.org/10.1038/nrn3320
Imai Y, Gehrke S, Wang H-Q, Takahashi R, Hasegawa K, Oota E, Lu B (2008) Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J 27:2432–2443. https://doi.org/10.1038/emboj.2008.163
Janusz A, Milek J, Perycz M, Pacini L, Bagni C, Kaczmarek L, Dziembowska M (2013) The Fragile × mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci 33:18234–18241. https://doi.org/10.1523/JNEUROSCI.2207-13.2013
Khaliq ZM, Bean BP (2010) Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci 30:7401–7413. https://doi.org/10.1523/JNEUROSCI.0143-10.2010
Khandjian EW, Corbin F, Woerly S, Rousseau F (1996) The fragile × mental retardation protein is associated with ribosomes. Nat Genet 12:91–93. https://doi.org/10.1038/ng0196-91
Kremer HP, Bots GT (1993) Lewy bodies in the lateral hypothalamus: do they imply neuronal loss? Mov Disord 8:315–320. https://doi.org/10.1002/mds.870080310
Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108. https://doi.org/10.1038/ng0298-106
Kthiri F, Gautier V, Le H-T, Prère M-F, Fayet O, Malki A, Landoulsi A, Richarme G (2010) Translational defects in a mutant deficient in YajL, the bacterial homolog of the parkinsonism-associated protein DJ-1. J Bacteriol 192:6302–6306. https://doi.org/10.1128/JB.01077-10
Lee HY, Ge W-P, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY (2011) Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile × mental retardation protein. Neuron 72:630–642. https://doi.org/10.1016/j.neuron.2011.09.033
Levin J, Giese A, Boetzel K, Israel L, Högen T, Nübling G, Kretzschmar H, Lorenzl S (2009) Increased alpha-synuclein aggregation following limited cleavage by certain matrix metalloproteinases. Exp Neurol 215:201–208. https://doi.org/10.1016/j.expneurol.2008.10.010
Lin W, Wadlington NL, Chen L, Zhuang X, Brorson JR, Kang UJ (2014) Loss of PINK1 attenuates HIF-1α induction by preventing 4E-BP1-dependent switch in protein translation under hypoxia. J Neurosci 34:3079–3089. https://doi.org/10.1523/JNEUROSCI.2286-13.2014
Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J (2005) K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci 8:1742–1751. https://doi.org/10.1038/nn1570
Liss B, Roeper J (2010) Ion channels and regulation of dopamine neuron activity. Sudha M Dopamine handbook. Oxford University Press, Oxford, pp 1–21
Liu S, Lu B (2010) Reduction of protein translation and activation of autophagy protect against PINK1 pathogenesis in Drosophila melanogaster. PLoS Genet 6:e1001237. https://doi.org/10.1371/journal.pgen.1001237
Martin I, Kim JW, Lee BD, Kang HC, Xu J-C, Jia H, Stankowski J, Kim M-S, Zhong J, Kumar M, Andrabi SA, Xiong Y, Dickson DW, Wszolek ZK, Pandey A, Dawson TM, Dawson VL (2014) Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell 157:472–485. https://doi.org/10.1016/j.cell.2014.01.064
Masi A, Narducci R, Resta F, Carbone C, Kobayashi K, Mannaioni G (2015) Differential contribution of Ih to the integration of excitatory synaptic inputs in substantia nigra pars compacta and ventral tegmental area dopaminergic neurons. Eur J Neurosci 42:2699–2706. https://doi.org/10.1111/ejn.13066
Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J (2003) RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37:417–431
Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, Beck S (2014) ChAMP: 450k chip analysis methylation pipeline. Bioinformatics 30:428–430. https://doi.org/10.1093/bioinformatics/btt684
Mutez E, Nkiliza A, Belarbi K, de Broucker A, Vanbesien-Mailliot C, Bleuse S, Duflot A, Comptdaer T, Semaille P, Blervaque R, Hot D, Leprêtre F, Figeac M, Destée A, Chartier-Harlin M-C (2014) Involvement of the immune system, endocytosis and EIF2 signaling in both genetically determined and sporadic forms of Parkinson’s disease. Neurobiol Dis 63:165–170. https://doi.org/10.1016/j.nbd.2013.11.007
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, Ikram MA, Ioannidis JPA, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB, International Parkinson’s Disease Genomics Consortium (IPDGC), Parkinson’s Study Group (PSG) Parkinson’s Research: The Organized GENetics Initiative (PROGENI), 23andMe, GenePD, NeuroGenetics Research Consortium (NGRC), Hussman Institute of Human Genomics (HIHG), Ashkenazi Jewish Dataset Investigator, Cohorts for Health and Aging Research in Genetic Epidemiology (CHARGE), North American Brain Expression Consortium (NABEC), United Kingdom Brain Expression Consortium (UKBEC), Greek Parkinson’s Disease Consortium, Alzheimer Genetic Analysis Group (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46:989–993. https://doi.org/10.1038/ng.3043
Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C (2008) The fragile × syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134:1042–1054. https://doi.org/10.1016/j.cell.2008.07.031
Niu Y-Q, Yang J-C, Hall DA, Leehey MA, Tassone F, Olichney JM, Hagerman RJ, Zhang L (2014) Parkinsonism in fragile X-associated tremor/ataxia syndrome (FXTAS): revisited. Parkinsonism Relat Disord 20:456–459. https://doi.org/10.1016/j.parkreldis.2014.01.006
Nordlund J, Bäcklin CL, Wahlberg P, Busche S, Berglund EC, Eloranta M-L, Flaegstad T, Forestier E, Frost B-M, Harila-Saari A, Heyman M, Jónsson OG, Larsson R, Palle J, Rönnblom L, Schmiegelow K, Sinnett D, Söderhäll S, Pastinen T, Gustafsson MG, Lönnerholm G, Syvänen A-C (2013) Genome-wide signatures of differential DNA methylation in pediatric acute lymphoblastic leukemia. Genome Biol 14:r105. https://doi.org/10.1186/gb-2013-14-9-r105
Osterweil EK, Chuang S-C, Chubykin AA, Sidorov M, Bianchi R, Wong RKS, Bear MF (2013) Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile × syndrome. Neuron 77:243–250. https://doi.org/10.1016/j.neuron.2012.01.034
Osterweil EK, Krueger DD, Reinhold K, Bear MF (2010) Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile × syndrome. J Neurosci 30:15616–15627. https://doi.org/10.1523/JNEUROSCI.3888-10.2010
Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B (1999) alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19:5782–5791
Ottone C, Galasso A, Gemei M, Pisa V, Gigliotti S, Piccioni F, Graziani F, Verrotti di Pianella A (2011) Diminution of eIF4E activity suppresses parkin mutant phenotypes. Gene 470:12–19. https://doi.org/10.1016/j.gene.2010.09.003
Pacelli C, Giguère N, Bourque M-J, Lévesque M, Slack RS, Trudeau L-É (2015) Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol 25:2349–2360. https://doi.org/10.1016/j.cub.2015.07.050
Pérez-Villalba A, Sirerol-Piquer MS, Belenguer G, Soriano-Cantón R, Muñoz-Manchado AB, Villadiego J, Alarcón-Arís D, Soria FN, Dehay B, Bezard E, Vila M, Bortolozzi A, Toledo-Aral JJ, Pérez-Sánchez F, Fariñas I (2018) Synaptic regulator α-synuclein in dopaminergic fibers is essentially required for the maintenance of subependymal neural stem cells. J Neurosci 38:814–825. https://doi.org/10.1523/JNEUROSCI.2276-17.2017
Philippart F, Destreel G, Merino-Sepulveda P, Henny P, Engel D, Seutin V (2016) Differential somatic Ca2+ channel profile in midbrain dopaminergic neurons. J Neurosci 36:7234–7245. https://doi.org/10.1523/JNEUROSCI.0459-16.2016
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Qin M (2005) Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the Fmr1 null mouse. J Neurosci 25:5087–5095. https://doi.org/10.1523/JNEUROSCI.0093-05.2005
Qin M, Schmidt KC, Zametkin AJ, Bishu S, Horowitz LM, Burlin TV, Xia Z, Huang T, Quezado ZM, Smith CB (2013) Altered cerebral protein synthesis in fragile × syndrome: studies in human subjects and knockout mice. J Cereb Blood Flow Metab 33:499–507. https://doi.org/10.1038/jcbfm.2012.205
Reyniers L, Del Giudice MG, Civiero L, Belluzzi E, Lobbestael E, Beilina A, Arrigoni G, Derua R, Waelkens E, Li Y, Crosio C, Iaccarino C, Cookson MR, Baekelandt V, Greggio E, Taymans J-M (2014) Differential protein-protein interactions of LRRK1 and LRRK2 indicate roles in distinct cellular signaling pathways. J Neurochem 131:239–250. https://doi.org/10.1111/jnc.12798
Richter JD, Bassell GJ, Klann E (2015) Dysregulation and restoration of translational homeostasis in fragile × syndrome. Nat Rev Neurosci 16:595–605. https://doi.org/10.1038/nrn4001
Riley BE, Gardai SJ, Emig-Agius D, Bessarabova M, Ivliev AE, Schüle B, Schüle B, Alexander J, Wallace W, Halliday GM, Langston JW, Braxton S, Yednock T, Shaler T, Johnston JA (2014) Systems-based analyses of brain regions functionally impacted in Parkinson’s disease reveals underlying causal mechanisms. PLoS One 9:e102909. https://doi.org/10.1371/journal.pone.0102909
Ronesi JA, Collins KA, Hays SA, Tsai N-P, Guo W, Birnbaum SG, Hu J-H, Worley PF, Gibson JR, Huber KM (2012) Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile × syndrome. Nat Neurosci 15:431. https://doi.org/10.1038/nn.3033
Routh BN, Johnston D, Brager DH (2013) Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile × syndrome. J Neurosci 33:19442–19450. https://doi.org/10.1523/JNEUROSCI.3256-13.2013
Scaglione C, Ginestroni A, Vella A, Dotti MT, Nave RD, Rizzo G, De Cristofaro MT, De Stefano N, Piacentini S, Martinelli P, Mascalchi M (2008) MRI and SPECT of midbrain and striatal degeneration in fragile X-associated tremor/ataxia syndrome. J Neurol 255:144–146. https://doi.org/10.1007/s00415-007-0711-8
Schmidt EK, Clavarino G, Ceppi M, Pierre P (2009) SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6:275–277. https://doi.org/10.1038/nmeth.1314
Scholz D, Pöltl D, Genewsky A, Weng M, Waldmann T, Schildknecht S, Leist M (2011) Rapid, complete and large-scale generation of post-mitotic neurons from the human LUHMES cell line. J Neurochem 119:957–971. https://doi.org/10.1111/j.1471-4159.2011.07255.x
Schulz J, Pagano G, Fernández Bonfante JA, Wilson H, Politis M (2018) Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain 141:1501–1516. https://doi.org/10.1093/brain/awy072
Sellier C, Buijsen RAM, He F, Natla S, Jung L, Tropel P, Gaucherot A, Jacobs H, Meziane H, Vincent A, Champy M-F, Sorg T, Pavlovic G, Wattenhofer-Donze M, Birling M-C, Oulad-Abdelghani M, Eberling P, Ruffenach F, Joint M, Anheim M, Martinez-Cerdeno V, Tassone F, Willemsen R, Hukema RK, Viville S, Martinat C, Todd PK, Charlet-Berguerand N (2017) Translation of expanded CGG repeats into FMRpolyG is pathogenic and may contribute to fragile × tremor ataxia syndrome. Neuron 93:331–347. https://doi.org/10.1016/j.neuron.2016.12.016
Sgobio C, Kupferschmidt DA, Cui G, Sun L, Li Z, Cai H, Lovinger DM (2014) Optogenetic measurement of presynaptic calcium transients using conditional genetically encoded calcium indicator expression in dopaminergic neurons. PLoS One 9:e111749. https://doi.org/10.1371/journal.pone.0111749
Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS (2010) Dysregulation of mTOR signaling in fragile × syndrome. J Neurosci 30:694–702. https://doi.org/10.1523/JNEUROSCI.3696-09.2010
Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM (2014) Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile × syndrome in a mouse model. J Neurosci 34:9867–9879. https://doi.org/10.1523/JNEUROSCI.1162-14.2014
Siller SS, Broadie K (2011) Neural circuit architecture defects in a Drosophila model of Fragile × syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis Model Mech 4:673–685. https://doi.org/10.1242/dmm.008045
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841. https://doi.org/10.1126/science.1090278
Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G (1993) The protein product of the fragile × gene, FMR1, has characteristics of an RNA-binding protein. Cell 74:291–298
Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:3–25. https://doi.org/10.2202/1544-6115.1027
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. https://doi.org/10.1038/42166
Stefani G, Fraser CE, Darnell JC, Darnell RB (2004) Fragile × mental retardation protein is associated with translating polyribosomes in neuronal cells. J Neurosci 24:7272–7276. https://doi.org/10.1523/JNEUROSCI.2306-04.2004
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. https://doi.org/10.1038/nrn.2016.178
Taha MS, Nouri K, Milroy LG, Moll JM, Herrmann C, Brunsveld L, Piekorz RP, Ahmadian MR (2014) Subcellular fractionation and localization studies reveal a direct interaction of the fragile × mental retardation protein (FMRP) with nucleolin. PLoS One 9:e91465. https://doi.org/10.1371/journal.pone.0091465
Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ (2009) Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci 12:1129–1135. https://doi.org/10.1038/nn.2372
Tamanini F, Meijer N, Verheij C, Willems PJ, Galjaard H, Oostra BA, Hoogeveen AT (1996) FMRP is associated to the ribosomes via RNA. Hum Mol Genet 5:809–813
Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S (2013) A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450k DNA methylation data. Bioinformatics 29:189–196. https://doi.org/10.1093/bioinformatics/bts680
Thannickal TC, Lai Y-Y, Siegel JM (2007) Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130:1586–1595. https://doi.org/10.1093/brain/awm097
Tian Y, Morris TJ, Webster AP, Yang Z, Beck S, Feber A, Teschendorff AE (2017) ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 33:3982–3984. https://doi.org/10.1093/bioinformatics/btx513
Udagawa T, Farny NG, Jakovcevski M, Kaphzan H, Alarcon JM, Anilkumar S, Ivshina M, Hurt JA, Nagaoka K, Nalavadi VC, Lorenz LJ, Bassell GJ, Akbarian S, Chattarji S, Klann E, Richter JD (2013) Genetic and acute CPEB1 depletion ameliorate fragile × pathophysiology. Nat Med 19:1473–1477. https://doi.org/10.1038/nm.3353
Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile × syndrome. Cell 65:905–914
Volpicelli-Daley LA, Kirik D, Stoyka LE, Standaert DG, Harms AS (2016) How can rAAV-α-synuclein and the fibril α-synuclein models advance our understanding of Parkinson’s disease? J Neurochem 139(Suppl 1):131–155. https://doi.org/10.1111/jnc.13627
Wang G, Achim CL, Hamilton RL, Wiley CA, Soontornniyomkij V (1999) Tyramide signal amplification method in multiple-label immunofluorescence confocal microscopy. Methods 18:459–464. https://doi.org/10.1006/meth.1999.0813
Wang H, Morishita Y, Miura D, Naranjo JR, Kida S, Zhuo M (2012) Roles of CREB in the regulation of FMRP by group I metabotropic glutamate receptors in cingulate cortex. Mol Brain 5:27. https://doi.org/10.1186/1756-6606-5-27
Waskiewicz AJ, Flynn A, Proud CG, Cooper JA (1997) Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 16:1909–1920. https://doi.org/10.1093/emboj/16.8.1909
Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA (1999) Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol 19:1871–1880
Winslow AR, Chen C-W, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC (2010) α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol 190:1023–1037. https://doi.org/10.1083/jcb.201003122
Yorgason JT, España RA, Jones SR (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202:158–164. https://doi.org/10.1016/j.jneumeth.2011.03.001
Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley JC, Warren ST, Schlessinger D (1991) Fragile × genotype characterized by an unstable region of DNA. Science 252:1179–1181
Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, Frick A (2014) Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(−/y) mice. Nat Neurosci 17:1701–1709. https://doi.org/10.1038/nn.3864
Zhou W, Laird PW, Shen H (2017) Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res 45:e22. https://doi.org/10.1093/nar/gkw967
(1994) Fmr1 knockout mice: a model to study fragile × mental retardation. The Dutch-Belgian fragile × consortium. Cell 78:23–33
Acknowledgements
This study was supported by the Parkinson Fonds Deutschland, the Hilde-Ulrichs-Stiftung, the Friede-Springer-Stiftung, and the Förderprogramm Forschung und Lehre (FöFoLe) , Ludwig Maximilian University, Munich, Germany (all to T.K.). Günter Höglinger was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy—ID 390857198), Deutsche Forschungsgemeinschaft (DFG, HO2402/18-1 MSAomics), the German Federal Ministry of Education and Research (BMBF, 01KU1403A EpiPD; 01EK1605A HitTau), the NOMIS foundation (FTLD project), the Parkinson Fonds Deutschland (Hypothesis-free compound screen, alpha-Synuclein fragments in PD). Jorg Tost was funded by the French National Agency for Reserach (ANR, ANR-13 - EPIG - 0003 - 02, EPIPD, and ANR-18-RAR3-0001-01, MSAomics). Jochen Herms was funded by DZNE and by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the framework of the Munich Cluster for Systems Neurology (SyNergy, EXC 2145 / ID 390857198). We thank the Munich Brain Bank for supplying brain tissue samples. We thank Prof. J. Behrends and Dr. D. Steinbrenner for their technical assistance with patch-clamp recording.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests related to this study.
Ethical and approval of animal experiments
This study was approved by the Ethics Commission at the Ludwig Maximilian University Munich, Germany. All experiments involving animals were approved by the local committee on animal welfare and the laws and regulations of the local government authorities.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, Y., Sgobio, C., Arzberger, T. et al. Loss of fragile X mental retardation protein precedes Lewy pathology in Parkinson’s disease. Acta Neuropathol 139, 319–345 (2020). https://doi.org/10.1007/s00401-019-02099-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-019-02099-5