Abstract
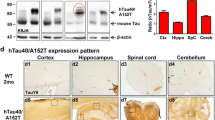
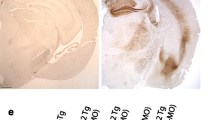
Mutations in the PARK2 gene encoding parkin cause autosomal recessive juvenile parkinsonism, but have also been found in patients diagnosed with certain tauopathies. Conversely, mutations in the MAPT gene encoding tau are present in some types of parkinsonism. In order to investigate the possible relationship between these two proteins, we generated a double mutant mouse that is deficient in PARK2 and that over-expresses the hTauVLW transgene, a mutant form of the tau protein present in FTDP-17. Independent deletion of PARK2 or over-expression of the hTauVLW transgene produces mild phenotypic alterations, while a substantial increase in parkin expression is observed in hTauVLW transgenic mice. However, double mutant mice present memory and exploratory deficits, and accumulation of PHF-1 and AT8 hyperphosphorylated tau epitopes in neurons. These phenomena are coupled with reactive astrocytosis, DNA fragmentation, and variable cerebral atrophy. Here, we show that cortical and hippocampal neurons of double mutant mice develop argyrophilic Gallyas-Braak aggregates of phosphorylated tau from 3 months of age. Their number decreases in old animals. Moreover, numerous phosphorylated tau aggregates were identified with the conformation-dependent Alz-50 antibody and the S-Thioflavin staining. Ventral motor nuclei of the spinal cord also present Alz-50, AT8, and PHF1 hyperphosphorylated tau aggregates when parkin is deleted in mice over-expressing the hTauVLW transgene, begining at early ages. Thus, the combination of PARK2 gene deletion with hTauVLW over-expression in mice produces abnormal hyperphosphorylated tau aggregates, similar to those observed in the brain of patients diagnosed with certain tauopathies. In the light of these changes, these mice may help to understand the molecular processes responsible for these diseases, and they may aid the development of new therapeutic strategies to treat neurodegenerative diseases related to tau and parkin proteins.





Similar content being viewed by others
References
Abbas N, Lucking CB, Ricard S et al (1999) A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson’s Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson’s Disease. Hum Mol Genet 8:567–574
Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98:6923–6928
Braak H, Braak E, Ohm T, Bohl J (1988) Silver impregnation of Alzheimer’s neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol 634:197–200
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089–1099
Engel T, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J, Hernandez F (2006) Co-expression of FTDP-17 tau and GSK-3β in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging 27:1258–1268
Feany MB, Dickson DW (1996) Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol 40:139–148
Gallyas F (1971) Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 19:1–8
Goedert M, Spillantini MG, Jakes R (1991) Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau. Neurosci Lett 126:149–154
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Nat Acad Sci USA 83:4913–4917
Guerrero R, Navarro P, Gallego E, Avila J, García de Yébenes J, Sanchez MP (2008) Park2-null/tau transgenic mice reveal a functional relationship between parkin and tau. J Alz Dis 13:161–172
Hattori N, Kitada T, Matsumine H et al (1998) Molecular genetic analysis of a novel Parkin gene in Japanese families with autosomal recessive juvenile parkinsonism: evidence for variable homozygous deletions in the Parkin gene in affected individuals. Ann Neurol 44:935–941
Hayashi S, Wakabayashi K, Ishikawa A et al (2000) An autopsy case of autosomal-recessive juvenile parkinsonism with a homozygous exon 4 deletion in the parkin gene. Mov Disord 15:884–888
Hutton M, Lendon CL, Rizzu P (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705
Iqbal K, Grundke-Iqbal I (2006) Discoveries of Tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. J Alz Dis 9:219–242
Itier JM, Ibanez P, Mena MA et al (2003) Parkin gene inactivation alters behavior and dopamine neurotransmission in the mouse. Hum Mol Genet 12:2277–2291
Kitada T, Asakawa S, Hattori N et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608
Klein RL, Dayton RD, Henderson KM, Petrucelli L (2006) Parkin is protective for substantia nigra dopamine neurons in a tau gene transfer neurodegeneration model. Neurosci Lett 401:130–135
Layfield R, Cavey JR, Lowe J (2003) Role of ubiquitin-mediated proteolysis in the pathogenesis of neurodegenerative disorders. Ageing Res Rev 2:343–356
Leroy E, Anastasopoulos D, Konitsiotis S, Laveda C, Polymeropoulos MH (1998) Deletions in the Parkin gene and genetic heterogeneity in a Greek family with early onset Parkinson’s disease. Hum Genet 103:424–427
Lim F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J (2001) FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Mol Cell Neurosci 8:702–714
Markopoulou K, Dickson DW, McComb RD, Wszolek ZK, Katechalidou L, Avery L, Stansbury MS, Chase BA (2008) Clinical, neuropatological and genotypic variability in SNCA A53T familial Parkinson’s disease. Variability in familial Parkinson’s disease. Acta Neuropathol 116:25–35
Matsumine H, Saito M, Shimoda-Matsubayashi S (1997) Localization of a gene for an autosomal recessive form of juvenile Parkinsonism to chromosome 6q25.2–27. Am J Hum Genet 60:588–596
Menendez J, Rodriguez-Navarro JA, Solano RM et al (2006) Suppression of Parkin enhances nigrostriatal and motor neuron lesion in mice over-expressing human-mutated tau protein. Hum Mol Genet 15:2045–2058
Morales B, Martinez A, Gonzalo I et al (2002) Steele-Richardson-Olszewski syndrome in a patient with a single C212Y mutation in the parkin protein. Mov Disord 7:1374–1380
Mori H, Kondo T, Yokochi M (1998) Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology 51:890–892
Moussa CE (2008) Parkin Attenuates Wild-Type tau Modification in the Presence of beta-Amyloid and alpha-Synuclein. J Mol Neurosci, 2008 Jun 17 (Epub ahead of print)
Navarro P, Guerrero R, Gallego E, Ávila J, Luquin R, García Ruíz PJ, Sánchez MP (2008) Memory and exploratory impairment in mice that lack the Park-2 gene and that over-express the human FTDP-17 mutant Tau. Behav Brain Res 189:350–356
Navarro P, Guerrero R, Gallego E, Ávila J, Luquin R, García Ruíz PJ, Sánchez MP (2008) Motor alterations are reduced in mice lacking the PARK2 gene in the presence of a human FTDP-17 mutant form of four-repeat tau. J Neurol Sci 275:139–144
O’Callaghan JP, Jensen KF (1992) Enhanced expression of glial fibrillary acidic protein and the cupric silver degeneration reaction can be used as sensitive and early indicators of neurotoxicity. Neurotoxicol 13:113–122
Petrucelli LK, Lockhart P (2002) Tau is a parkin substrate; implications for neurodegenerative disease. Mov Disord 17:S156
Petrucelli L, Dickson D, Kehoe K et al (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13:703–714
Poorkaj P, Bird TD, Wijsman E (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825
Ren Y, Zhao J, Feng J (2003) Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci 23:3316–3324
Sahara N, Murayama M, Mizoroki T et al (2005) In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem 94:1254–1263
Sánchez MP, Gonzalo I, Avila J, García de Yébenes J (2002) Progressive supranuclear palsy and tau hyperphosphorylation in a patient with a C212Y parkin mutation. J Alz Dis 4:399–404
Shimura H, Hattori N, Kubo S (2000) Familial Parkinson disease gene product, parkin, is an ubiquitin-protein ligase. Nat Genet 25:302–305
Shimura H, Schwartz D, Gygi SP, Kosik KS (2004) CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem 279:4869–4876
Spillantini MG (2001) Tau and Parkinson disease. JAMA 286:2324–2326
Spillantini MG, Bird TD, Ghetti B (1998) Frontotemporal dementia and Parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol 8:387–402
Stanford PM, Brooks WS, Teber ET (2004) Frequency of tau mutations in familial and sporadic frontotemporal dementia and other tauopathies. J Neurol 251:1098–1104
van De Warrenburg BP, Lammens M, Lucking CB (2001) Clinical and pathologic abnormalities in a family with parkinsonism and parkin gene mutations. Neurology 56:555–557
van Leeuwen FW, Hol EM, Fischer DF (2006) Frameshift proteins in Alzheimer’s disease and in other conformational disorders: Time for the ubiquitin-proteasome system. J Alz Dis 9:319–325
von Coelln R, Dawson VL, Dawson TM (2004) Parkin-associated Parkinson’s disease. Cell Tissue Res 318:175–184
West A, Lincoln S, Lucking CB et al (2002) French. Parkinson’s Disease Genetics Study Group and the European Consortium on Genetic Susceptibility on Parkinson’s Disease. Complex relationship between Parkin mutations and Parkinson disease. Am J Med Genet 114:584–591
Wolozin BL, Pruchnicki A, Dickson DB, Davies P (1986) A neuronal antigen in the brains of Alzheimer patients. Science 232:648–650
Wszolek ZK, Pfeiffer RF, Tsuboi Y et al (2004) Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology 62:1619–1622
Yang F, Jiang Q, Zhao J, Ren Y, Sutton MD, Feng J (2005) Parkin stabilizes microtubules through strong binding mediated by three independent domains. J Biol Chem 280:17154–17162
Acknowledgments
The authors would like to thank Drs Jeremy Park, Jesus Benavides and Filip Lim for the use of the Park−/− and TauVLW mice; Drs Francisco Wandosell and Jose Javier Lucas for critical reading of the manuscript and for helpful discussions; and Dr. Jose Luis Sarasa for supervising the neuropathological analysis. They are also grateful to Izaskun Rodal for technical support and to the Animal Facility of the Fundación Jiménez Díaz-Capio. This work was supported by grants from the Fondo de Investigaciones Sanitarias (PI030165 and PI070023) from the Spanish Ministry of Health; P.N. and A.M.G.C. were supported by fellowships from the Fundación Conchita Rábago. Dr. Avila acts as an advisor for Neuropharma (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerrero, R., Navarro, P., Gallego, E. et al. Hyperphosphorylated tau aggregates in the cortex and hippocampus of transgenic mice with mutant human FTDP-17 Tau and lacking the PARK2 gene. Acta Neuropathol 117, 159–168 (2009). https://doi.org/10.1007/s00401-008-0470-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0470-3