Abstract
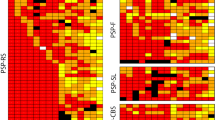
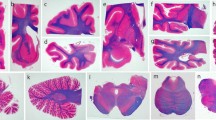
The deposition of abnormal levels of tau protein is a major neuropathological feature of progressive supranuclear palsy (PSP), and the presence of tuft-shaped astrocytes is a neuropathological hallmark of PSP. We examined the topographic distribution of tuft-shaped astrocytes in the cerebral hemisphere by Gallyas-Braak silver staining in three Japanese autopsy cases of typical PSP. The distribution of tuft-shaped astrocytes was relatively uniform between cases. Tuft-shaped astrocytes were identified predominantly in posterior frontal areas such as the precentral gyrus and premotor and supplementary motor areas (Brodmann areas 4, 6 and 8). Tuft-shaped astrocytes were most dense in areas of cortical convexity, and they were more abundant in the crests of the cerebral gyri than in the valleys of the cerebral sulci. The temporal, parietal and occipital cortices, including the hippocampal formation and cingulate gyrus, were relatively free of tuft-shaped astrocytes. We confirmed involvement of the cerebral cortex in the pathology of PSP, and showed the widespread presence of tuft-shaped astrocytes, particularly in the precentral gyrus and premotor and supplementary motor areas, to be an essential neuropathological feature of PSP. The extra-pyramidal and pyramidal signs, supranuclear oculomotor abnormalities and other cortical signs associated with PSP may be related to the high density of tuft-shaped astrocytes in the precentral gyrus and premotor and supplementary motor areas. Dementia, apraxia, aphasia and frontal lobe signs may also result, at least in part, from this cortical involvement.



Similar content being viewed by others
References
Agid Y, Javoy-Agid F, Ruberg M, Pillon B, Dubois B, Duyckaerts C, Hauw JJ, Baron JC, Scatton B (1987) Progressive supranuclear palsy: anatomoclinical and biochemical considerations. Adv Neurol 45:191–206
Akashi T, Arima K, Maruyama N, Ando S, Inose T (1989) Severe cerebral atrophy in progressive supranuclear palsy: a case report. Clin Neuropathol 8:195–199
Bergeron C, Pollanen MS, Weyer L, Lang AE (1997) Cortical degeneration in progressive supranuclear palsy. A comparison with cortical-basal ganglionic degeneration. J Neuropathol Exp Neurol 56:726–734
Bigio EH, Brown DF, White CL 3rd (1999) Progressive supranuclear palsy with dementia: cortical pathology. J Neuropathol Exp Neurol 58:359–364
Braak H, Braak E, Ohm T, Bohl J (1988) Silver impregnation of Alzheimer’s neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol 63:197–200
Daniel SE, Bruin VMS de, Lees AJ (1995) The clinical and pathological spectrum of Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy): a reappraisal. Brain 118:759–770
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246 [Suppl 2]:II6–II15
Duvoison RC (1995) Progressive supranuclear palsy. In: Rowland LP (ed) Merritt’s textbook of neurology, 9th edn. Williams & Wilkins, Baltimore, pp 730–733
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Hanihara T, Amano N, Takahashi T, Nagatomo H, Yagishita S (1995) Distribution of tangles and threads in the cerebral cortex in progressive supranuclear palsy. Neuropathol Appl Neurobiol 21:319–326
Hattori M, Hashizume Y, Yoshida M, Iwasaki Y, Hishikawa N, Ueda R, Ojika K (2003) Distribution of astrocytic plaques in the corticobasal degeneration brain and comparison with tuft-shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol 106:143–149
Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C (1990) Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer’s disease and aging. Neurosci Lett 119:182–186
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathological criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hof PR, Delacourte A, Bouras C (1992) Distribution of cortical neurofibrillary tangles in progressive supranuclear palsy: a quantitative analysis of six cases. Acta Neuropathol 84:45–51
Ikeda K (1996) Glial fibrillary tangles and argyrophilic threads: classification and disease specificity. Neuropathology 16:71–77
Ikeda K, Akiyama H, Kondo H, Haga C, Tanno E, Tokuda T, Ikeda S (1995) Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol 90:620–625
Ishino H, Otsuki S (1976) Frequency of Alzheimer’s neurofibrillary tangles in the cerebral cortex in progressive supranuclear palsy (subcortical argyrophilic dystrophy). J Neurol Sci 28:309–316
Komori T, Arai N, Oda M, Nakayama H, Mori H, Yagishita S, Takahashi T, Amano N, Murayama S, Murakami S, Shibata N, Kobayashi M, Sasaki S, Iwata M (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 96:401–408
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9
Lowe JS, Leigh N (2002) Disorder of movement and system degenerations. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, 7th edn. Arnold, London, pp 337–342
Matsusaka H, Ikeda K, Akiyama H, Arai T, Inoue M, Yagishita S (1998) Astrocytic pathology in progressive supranuclear palsy: significance for neuropathological diagnosis. Acta Neuropathol 96:248–252
Mochizuki A, Ueda Y, Komatsuzaki Y, Tsuchiya K, Arai T, Shoji S (2003) Progressive supranuclear palsy presenting with primary progressive aphasia-clinicopathological report of an autopsy case. Acta Neuropathol 105:610–614
Nishimura T, Ikeda K, Akiyama H, Kondo H, Kato M, Li F, Iseki E, Kosaka K (1995) Immunohistochemical investigation of tau-positive structures in the cerebral cortex of patients with progressive supranuclear palsy. Neurosci Lett 201:123–126
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Takahashi H, Takeda S, Ikuta F, Homma Y (1987) Progressive supranuclear palsy with limbic system involvement: report of a case with ultrastructural investigation of neurofibrillary tangles in various locations. Clin Neuropathol 6:271–276
Takeuchi T, Shibayama H, Iwai K, Iwata H, Xu M, Kitoh J, Kobayashi H, Iwase S, Nakagawa M, Yamada K, Yoshida K, Nambu Y, Ishihara R (1992) Progressive supranuclear palsy with widespread cerebral lesions. Clin Neuropathol 11:304–311
Togo T, Dickson DW (2002) Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol 104:398–402
Verny M, Duyckaerts C, Agid Y, Hauw JJ (1996) The significance of cortical pathology in progressive supranuclear palsy. Clinico-pathological data in 10 cases. Brain 119:1123–1136
Yamada T, McGeer PL, McGeer EG (1992) Appearance of paired nucleated, tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 135:99–102
Yamada T, Calne DB, Akiyama H, McGeer EG, McGeer PL (1993) Further observations on tau-positive glia in the brains with progressive supranuclear palsy. Acta Neuropathol 85:308–315
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwasaki, Y., Yoshida, M., Hattori, M. et al. Distribution of tuft-shaped astrocytes in the cerebral cortex in progressive supranuclear palsy. Acta Neuropathol 108, 399–405 (2004). https://doi.org/10.1007/s00401-004-0904-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0904-5