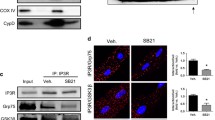
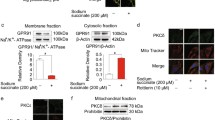
Abstract
Although mitochondria are key determinants of myocardial injury during ischemia–reperfusion (I/R), their interaction with critical cytoprotective signaling systems is not fully understood. Sphingosine-1-phosphate (S1P) produced by sphingosine kinase-1 protects the heart from I/R damage. Recently a new role for mitochondrial S1P produced by a second isoform of sphingosine kinase, SphK2, was described to regulate complex IV assembly and respiration via interaction with mitochondrial prohibitin-2. Here we investigated the role of SphK2 in cardioprotection by preconditioning. Littermate (WT) and sphk2 −/− mice underwent 45 min of in vivo ischemia and 24 h reperfusion. Mice received no intervention (I/R) or preconditioning (PC) via 5 min I/R before the index ischemia. Despite the activation of PC-cytoprotective signaling pathways in both groups, infarct size in sphk2 −/− mice was not reduced by PC (42 ± 3% PC vs. 43 ± 4% I/R, p = ns) versus WT (24 ± 3% PC vs. 43 ± 3% I/R, p < 0.05). sphk2 −/− mitochondria exhibited decreased oxidative phosphorylation and increased susceptibility to permeability transition (PTP). Unlike WT, PC did not prevent ischemic damage to electron transport or the increased susceptibility to PTP. To evaluate the direct contribution to the resistance of mitochondria to cytoprotection, SphK2, PHB2 or cytochrome oxidase subunit IV was depleted in cardiomyoblasts. PC protection was abolished by each knockdown concomitant with decreased PTP resistance. These results point to a new action of S1P in cardioprotection and suggest that the mitochondrial S1P produced by SphK2 is required for the downstream protective modulation of PTP as an effector of preconditioning protection.







Similar content being viewed by others
References
Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, Cacciatore F, Longobardi G, Rengo F (1996) Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol 27:1777–1786. doi:10.1016/0735-1097(96)00070-8
Argaud L, Gateau-Roesch O, Augeul L, Couture-Lepetit E, Loufouat J, Gomez L, Robert D, Ovize M (2008) Increased mitochondrial calcium coexists with decreased reperfusion injury in postconditioned (but not preconditioned) hearts. Am J Physiol Heart Circ Physiol 294:H386–H391. doi:10.1152/ajpheart.01035.2007
Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M (2004) Preconditioning delays Ca2+ -induced mitochondrial permeability transition. Cardiovasc Res 61:115–122. doi:10.1016/j.cardiores.2003.11.003
Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M (2005) Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. Journal of Molecular and Cellular Cardiology 38:367–374. doi:10.1016/j.yjmcc.2004.12.001
Beutner G, Ruck A, Riede B, Brdiczka D (1998) Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta 1368:7–18. doi:10.1016/S0005-2736(97)00175-2
Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R (2008) Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102:131–135. doi:10.1161/CIRCRESAHA.107.164699
Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R (2008) The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120:172–185. doi:10.1016/j.pharmthera.2008.08.002
Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R (2010) Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 105:771–785. doi:10.1007/s00395-010-0124-1
Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ (2007) Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol 292:C137–C147. doi:10.1152/ajpcell.00270.2006
Chen Q, Hoppel CL, Lesnefsky EJ (2006) Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther 316:200–207. doi:10.1124/jpet.105.091702
Chen Q, Lesnefsky EJ (2011) Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett 585:921–926. doi:10.1016/j.febslet.2011.02.029
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294:C460–C466. doi:10.1152/ajpcell.00211.2007
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2006) Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319:1405–1412. doi:10.1124/jpet.106.110262
Chen Q, Yin G, Stewart S, Hu Y, Lesnefsky EJ (2010) Isolating the segment of the mitochondrial electron transport chain responsible for mitochondrial damage during cardiac ischemia. Biochem Biophys Res Commun 397:656–660. doi:10.1016/j.bbrc.2010.05.137
Cohen MV, Yang XM, Downey JM (2008) Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning’s success. Basic Res Cardiol 103:464–471. doi:10.1007/s00395-008-0737-9
Davidson SM, Hausenloy D, Duchen MR, Yellon DM (2006) Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol 38:414–419. doi:10.1016/j.biocel.2005.09.017
Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL (1999) Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys 372:399–407. doi:10.1006/abbi.1999.1508
Gateau-Roesch O, Argaud L, Ovize M (2006) Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res 70:264–273. doi:10.1016/j.cardiores.2006.02.024
Gimenez-Cassina A, Lim F, Cerrato T, Palomo GM, Diaz-Nido J (2009) Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem 284:3001–3011. doi:10.1074/jbc.M808698200
Gomez L, Chavanis N, Argaud L, Chalabreysse L, Gateau-Roesch O, Ninet J, Ovize M (2005) Fas-independent mitochondrial damage triggers cardiomyocyte death after ischemia–reperfusion. Am J Physiol Heart Circ Physiol 289:H2153–H2158. doi:10.1152/ajpheart.00165.2005
Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M (2008) Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation 117:2761–2768. doi:10.1161/CIRCULATIONAHA.107.755066
Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M (2007) Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol 293:H1654–H1661. doi:10.1152/ajpheart.01378.2006
Gross ER, Hsu AK, Gross GJ (2006) The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3beta. Am J Physiol Heart Circ Physiol 291:H827–H834. doi:10.1152/ajpheart.00003.2006
Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R (1998) Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol 275:H1375–H1387
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325:1254–1257. doi:10.1126/science.1176709
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61:372–385. doi:10.1016/S0008-6363(03)00533-9
Hausenloy D, Wynne A, Duchen M, Yellon D (2004) Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 109:1714–1717. doi:10.1161/01.CIR.0000126294.81407.7D
Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM (2010) Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol 105:677–686. doi:10.1007/s00395-010-0121-4
Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM (2002) Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 55:534–543. doi:10.1016/S0008-6363(02)00455-8
Heusch G, Boengler K, Schulz R (2008) Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118:1915–1919. doi:10.1161/CIRCULATIONAHA.108.805242
Heusch G, Boengler K, Schulz R (2010) Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 105:151–154. doi:10.1007/s00395-009-0080-9
Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL (1994) Mitochondrial calcium spiking: a transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett 348:211–215. doi:10.1016/0014-5793(94)00615-6
Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S (2003) Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem 278:46832–46839. doi:10.1074/jbc.M30657720
Javadov SA (2003) Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol (Lond.) 549:513–524. doi:10.1113/jphysiol.2003.03423
Jin ZQ, Goetzl EJ, Karliner JS (2004) Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation 110:1980–1989. doi:10.1161/01.CIR.0000143632.06471.93
Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO (2002) Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol 282:H1970–H1977. doi:10.1152/ajpheart.01029.2001
Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ (2004) Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113:1535–1549. doi:10.1172/JCI19906
Karliner JS (2009) Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol 53:189–197. doi:10.1097/FJC.0b013e3181926706
Katoh H, Nishigaki N, Hayashi H (2002) Diazoxide opens the mitochondrial permeability transition pore and alters Ca2+ transients in rat ventricular myocytes. Circulation 105:2666–2671. doi:10.1161/01.CIR.0000016831.41648.04
Kelly RF, Lamont KT, Somers S, Hacking D, Lacerda L, Thomas P, Opie LH, Lecour S (2010) Ethanolamine is a novel STAT-3 dependent cardioprotective agent. Basic Res Cardiol 105:763–770. doi:10.1007/s00395-010-0125-0
Korge P, Ping P, Weiss JN (2008) Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res 103:873–880. doi:10.1161/CIRCRESAHA.108.180869
Lacerda L, McCarthy J, Mungly SF, Lynn EG, Sack MN, Opie LH, Lecour S (2010) TNFalpha protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol 105:751–762. doi:10.1007/s00395-010-0113-4
Lim SY, Davidson SM, Hausenloy DJ, Yellon DM (2007) Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res 75:530–535. doi:10.1016/j.cardiores.2007.04.022
Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S (2003) Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278:40330–40336. doi:10.1074/jbc.M30445520
Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH Jr, Milstien S, Spiegel S (2005) SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem 280:37118–37129. doi:10.1074/jbc.M502207200
Miura T, Tanno M, Sato T (2010) Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc Res 88:7–15. doi:10.1093/cvr/cvq206
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R (2010) Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 87:406–423. doi:10.1093/cvr/cvq129
Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E (1997) Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS Lett 406:136–138. doi:10.1016/S0014-5793(97)00264-0
Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F (1999) Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76:725–734. doi:10.1016/S0006-3495(99)77239-5
Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M (2008) Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359:473–481
Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA (2005) Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med 201:49–54. doi:10.1084/jem.20040559
Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B (2010) Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol 105:821–832. doi:10.1007/s00395-010-0112-5
Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G (2007) Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev 12:261–266
Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G (2002) No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol 283:H1740–H1742. doi:10.1152/ajpheart.00442.2002
Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S (2011) Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. Faseb J 25(2):600–612. doi:10.1096/fj.10-167502
Suleman N, Somers S, Smith R, Opie LH, Lecour SC (2008) Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res 79:127–133. doi:10.1093/cvr/cvn067
Tani M, Honma Y, Hasegawa H, Tamaki K (2001) Direct activation of mitochondrial K(ATP) channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res 49:56–68. doi:10.1016/S0008-6363(00)00240-6
Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B (2006) High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 114:1403–1409. doi:10.1161/CIRCULATIONAHA.105.607135
Vessey DA, Kelley M, Li L, Huang Y (2009) Sphingosine protects aging hearts from ischemia/reperfusion injury: superiority to sphingosine 1-phosphate and ischemic pre- and post-conditioning. Oxid Med Cell Longev 2:146–151
Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS (2006) Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit 12:BR 318–BR 324
Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, Karliner JS (2011) A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev 2011:8. doi:10.1155/2011/961059
Vessey DA, Li L, Kelley M, Karliner JS (2008) Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun 375:425–429. doi:10.1016/j.bbrc.2008.08.022
Vivaldi MT, Kloner RA, Schoen FJ (1985) Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol 121:522–530
Wacker BK, Park TS, Gidday JM (2009) Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke 40:3342–3348. doi:10.1161/STROKEAHA.109.560714
Yellon DM, Alkhulaifi AM, Pugsley WB (1993) Preconditioning the human myocardium. Lancet 342:276–277. doi:10.1016/0140-6736(93)91819-8
Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO (2007) Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293:H3150–H3158. doi:10.1152/ajpheart.00587.2006
Zhao ZQ, Vinten-Johansen J (2006) Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res 70:200–211. doi:10.1016/j.cardiores.2006.01.024
Acknowledgments
The authors thank Drs. Anindita Das and Robert Paillard for their advice and technical help. We thank Dr. Fadi N. Salloum from the Division of Cardiology, Department of Internal Medicine, Virginia Commonwealth University for echocardiographic measurements. And we thank Dr. Claudine Kieda from the Centre Biophysique Moléculaire (CNRS, France) for the gift of H9c2 cells. This work was supported by U.S. National Institutes of Health (NIH) grants 2PO1AG15885 (E.J.L.) and R37GM043880 (S.S), Medical Research Service, Department of Veterans Affairs Merit Review Award to E.J.L. and the Pauley Heart Center, Virginia Commonwealth University (E.J.L., Q.C., L.G., M.P.). L.G was supported by SERVIER grant “jeune chercheur” (France) and M.P. was supported by French grant EXPLORA’DOC 2009 (Rhône-Alpes, France).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2011_223_MOESM1_ESM.ppt
Supplemental Figure : Histological assessment of fibrosis in WT and sphk2 −/− mice. Histopathology of heart sections stained with Masson’s trichrome showing no significant myofiber disarray and the absence of interstitial fibrosis (stained blue) in WT and sphk2 −/− mice at 10 weeks of age. Transgenic mice do not demonstrate increased fibrosis compared to WT at 10 months of age (n = 2/group) (PPT 266 kb)
395_2011_223_MOESM2_ESM.ppt
Supplemental Table 1: Cardiac phenotype of sphk2 −/− mice. Echocardiography was performed with light anesthesia (pentobarbital 60 mg/kg IP). Images were acquired with a 13-MHz linear-array transducer with a digital ultrasound system (Vivid 7, GE Medical Systems, Waukesha, Wis). Conventional measurements were performed in WT vs sphk2 −/− mice at 15 weeks old: LV weight, heart rate, LV anterior (Aw) and posterior wall (Pw) thickness, LV dimensions (end-diastolic LVEDD and end-systolic LVESD diameters and interventricular septal diameter), and LV shortening fraction. Data are expressed as the mean ± SEM. Numerical data were compared using Student’s t test. Statistical significance was defined as a value of p<0.05. At baseline, WT and sphk2 −/− mice had comparable heart rates. LV end-systolic, LV end-diastolic diameters, wall thickness and shortening fractions were similar in two groups (p = ns, n = 4/groups) (PPT 120 kb)
Rights and permissions
About this article
Cite this article
Gomez, L., Paillard, M., Price, M. et al. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol 106, 1341–1353 (2011). https://doi.org/10.1007/s00395-011-0223-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-011-0223-7