Abstract
Purpose
Adipose tissue is central to the regulation of energy balance. Two functionally different fat pads are present in mammals: white adipose tissue, the primary site of triglyceride storage, and brown adipose tissue (BAT), which is specialized in heat production. In this context, new strategies capable of modulating the development and function of white and BAT become relevant. In the present study, we analyzed the influence of resveratrol (sirtuin activator) on energy balance and the expression of thermogenesis markers.
Methods
Mice were divided into two groups: standard diet (ST) and standard diet plus resveratrol (ST + RSV).
Results
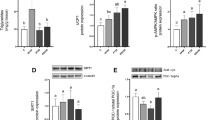
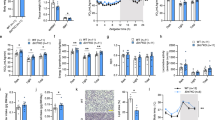
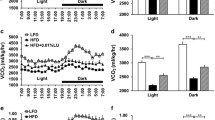
After 2 months of treatment, ST + RSV mice presented significantly decreased fat accumulation in adipose tissue, with diminished total cholesterol and glucose plasma levels. Additionally, increased oxygen consumption was observed in ST + RSV group. Analyses of mRNA of thermogenesis-related genes showed significant increase in UCP1, SIRT1, PTEN and BMP-7 expression in BAT.
Conclusion
Our data suggest that improved metabolism produced by oral administration of resveratrol is, at least in part, associated with increased thermogenesis followed by high expression of UCP1 and SIRT1, which can mediate higher energy expenditure and decreased fat accumulation in adipose tissue.




Similar content being viewed by others
References
Gesta S, Tseng YH, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131(2):242–256. doi:10.1016/j.cell.2007.10.004
Del Gonzalez-BarrosoM M, Ricquier D, Cassard-Doulcier AM (2000) The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obes Rev 1(2):61–72
Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP (2013) Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem 141(2):1530–1535. doi:10.1016/j.foodchem.2013.03.085
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–342. doi:10.1038/nature05354
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5(6):493–506. doi:10.1038/nrd2060
Carafa V, Nebbioso A, Altucci L (2012) Sirtuins and disease: the road ahead. Front Pharmacol 3:4. doi:10.3389/fphar.2012.00004
Lavu S, Boss O, Elliott PJ, Lambert PD (2008) Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7(10):841–853. doi:10.1038/nrd2665
Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Arias N, Andres-Lacueva C, Portillo MP (2011) Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr Metab (Lond) 8(1):29. doi:10.1186/1743-7075-8-29
Kim S, Jin Y, Choi Y, Park T (2011) Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81(11):1343–1351. doi:10.1016/j.bcp.2011.03.012
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D (2012) Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 150(3):620–632. doi:10.1016/j.cell.2012.06.027
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. doi:10.1038/nature07813
Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450(7170):712–716. doi:10.1038/nature06261
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127(6):1109–1122. doi:10.1016/j.cell.2006.11.013
de Pinho L, Andrade JM, Paraiso A, Filho AB, Feltenberger JD, Guimaraes AL, de Paula AM, Caldeira AP, Botelho AC, Campagnole-Santos MJ, Santos SH (2013) Diet composition modulate expression of sirtuins and renin-angiotensin system components in adipose tissue. Obesity. doi:10.1002/oby.20305
Feltenberger JD, Andrade JM, Paraiso A, Barros LO, Filho AB, Sinisterra RD, Sousa FB, Guimaraes AL, de Paula AM, Campagnole-Santos MJ, Qureshi M, Dos Santos RA, Santos SH (2013) Oral formulation of angiotensin-(1–7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension. doi:10.1161/HYPERTENSIONAHA.111.00919
Leite LH, Lacerda AC, Balthazar CH, Marubayashi U, Coimbra CC (2007) Central AT(1) receptor blockade increases metabolic cost during exercise reducing mechanical efficiency and running performance in rats. Neuropeptides 41(3):189–194. doi:10.1016/j.npep.2007.01.002
Guimaraes JB, Wanner SP, Machado SC, Lima MR, Cordeiro LM, Pires W, La Guardia RB, Silami-Garcia E, Rodrigues LO, Lima NR (2013) Fatigue is mediated by cholinoceptors within the ventromedial hypothalamus independent of changes in core temperature. Scand J Med Sci Sports 23(1):46–56. doi:10.1111/j.1600-0838.2011.01350.x
de Queiroz KB, Rodovalho GV, Guimaraes JB, de Lima DC, Coimbra CC, Evangelista EA, Guerra-Sa R (2012) Endurance training blocks uncoupling protein 1 up-regulation in brown adipose tissue while increasing uncoupling protein 3 in the muscle tissue of rats fed with a high-sugar diet. Nutr Res 32(9):709–717. doi:10.1016/j.nutres.2012.06.020
Saely CH, Geiger K, Drexel H (2012) Brown versus white adipose tissue: a mini-review. Gerontology 58(1):15–23. doi:10.1159/000321319
Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK (2011) Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011:490650. doi:10.1155/2011/490650
Caesar R, Manieri M, Kelder T, Boekschoten M, Evelo C, Muller M, Kooistra T, Cinti S, Kleemann R, Drevon CA (2010) A combined transcriptomics and lipidomics analysis of subcutaneous, epididymal and mesenteric adipose tissue reveals marked functional differences. PLoS ONE 5(7):e11525. doi:10.1371/journal.pone.0011525
DiGirolamo M, Fine JB, Tagra K, Rossmanith R (1998) Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am J Physiol 274(5 Pt 2):R1460–R1467
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA (2013) The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. doi:10.1111/febs.12619
Bartelt A, Heeren J (2014) Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10(1):24–36. doi:10.1038/nrendo.2013.204
Giralt M, Villarroya F (2013) White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154(9):2992–3000. doi:10.1210/en.2013-1403
Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP (2003) Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Investig 111(3):399–407. doi:10.1172/JCI15737
Hamann A, Flier JS, Lowell BB (1996) Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 137(1):21–29
Okamatsu-Ogura Y, Nio-Kobayashi J, Iwanaga T, Terao A, Kimura K, Saito M (2011) Possible involvement of uncoupling protein 1 in appetite control by leptin. Exp Biol Med 236(11):1274–1281. doi:10.1258/ebm.2011.011143
Oh HH, Kim KS, Choi SM, Yang HS, Yoon Y (2004) The effects of uncoupling protein-1 genotype on lipoprotein cholesterol level in Korean obese subjects. Metab Clin Exp 53(8):1054–1059
Sale MM, Hsu FC, Palmer ND, Gordon CJ, Keene KL, Borgerink HM, Sharma AJ, Bergman RN, Taylor KD, Saad MF, Norris JM (2007) The uncoupling protein 1 gene, UCP1, is expressed in mammalian islet cells and associated with acute insulin response to glucose in African American families from the IRAS Family Study. BMC Endocr Disord 7:1. doi:10.1186/1472-6823-7-1
Proenza AM, Poissonnet CM, Ozata M, Ozen S, Guran S, Palou A, Strosberg AD (2000) Association of sets of alleles of genes encoding beta3-adrenoreceptor, uncoupling protein 1 and lipoprotein lipase with increased risk of metabolic complications in obesity. Int J Obes Relat Metab Disord J Int Assoc Study Obes 24(1):93–100
Shin HD, Kim KS, Cha MH, Yoon Y (2005) The effects of UCP-1 polymorphisms on obesity phenotypes among Korean female subjects. Biochem Biophys Res Commun 335(2):624–630. doi:10.1016/j.bbrc.2005.07.096
Jia JJ, Tian YB, Cao ZH, Tao LL, Zhang X, Gao SZ, Ge CR, Lin QY, Jois M (2010) The polymorphisms of UCP1 genes associated with fat metabolism, obesity and diabetes. Mol Biol Rep 37(3):1513–1522. doi:10.1007/s11033-009-9550-2
Kozak LP, Harper ME (2000) Mitochondrial uncoupling proteins in energy expenditure. Annu Rev Nutr 20:339–363. doi:10.1146/annurev.nutr.20.1.339
Lanouette CM, Giacobino JP, Perusse L, Lacaille M, Yvon C, Chagnon M, Kuhne F, Bouchard C, Muzzin P, Chagnon YC (2001) Association between uncoupling protein 3 gene and obesity-related phenotypes in the Quebec Family Study. Mol Med 7(7):433–441
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98(1):115–124. doi:10.1016/S0092-8674(00)80611-X
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434(7029):113–118. doi:10.1038/nature03354
Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Munoz-Martin M, Gomez-Lopez G, Canamero M, Mulero F, Pastor J, Martinez S, Romanos E, Mar Gonzalez-Barroso M, Rial E, Valverde AM, Bischoff JR, Serrano M (2012) Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab 15(3):382–394. doi:10.1016/j.cmet.2012.02.001
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454(7207):1000–1004. doi:10.1038/nature07221
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM (2007) Transcriptional control of brown fat determination by PRDM16. Cell Metab 6(1):38–54. doi:10.1016/j.cmet.2007.06.001
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839
Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH (2013) Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495(7441):379–383. doi:10.1038/nature11943
Acknowledgments
This work was partially supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq) and Minas Gerais State Foundation for Research Development (FAPEMIG).
Conflict of interest
There is no conflict of interest to disclose for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
João Marcus Oliveira Andrade and Alessandra Caroline Montes Frade equally contributed to this study.
Rights and permissions
About this article
Cite this article
Andrade, J.M.O., Frade, A.C.M., Guimarães, J.B. et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr 53, 1503–1510 (2014). https://doi.org/10.1007/s00394-014-0655-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0655-6