Abstract
Previous studies have demonstrated the anti-inflammatory effect of fructooligosaccharides (FOS) on intestinal inflammation. The aim of the present study was to elucidate whether the colonic fermentation of these carbohydrates is a pre-requisite for this anti-inflammatory activity.
With this aim short chain-FOS (SC-FOS) were used for an in vitro fermentation to elucidate the time of fermentation of these compounds. For the in vivo experiments female Wistar rats were fed several diets with different sources of fibre (5 g/kg): cellulose for control rats (n = 30) or SC-FOS (n = 20) with a high content of kestose (GF2) for the SC-FOS group. After one month of feeding the different diets 10 rats from each group were sacrificed to analyze cecal and colonic microflora, SCFA production and pH of intestinal contents. A distal colonic inflammation was induced to other 10 rats from each group by the administration of 10 mg of TNBS dissolved in 0.25 ml of 50% ethanol (v/v). The rest of the rats from the control group (n = 10) were rendered healthy. One week after TNBS treatment rats were sacrificed and several inflammatory parameters as well as intestinal microbiota and SCFA contents were analyzed.
In vitro fermentation experiments showed that SC-FOS are fermented during the first 12 h after incorporating the oligosaccharides to intestinal contents, thus suggesting a preferential fermentation of these carbohydrates in the ileum and cecum. In fact, SC-FOS increased cecal lactobacilli and bifidobacteria counts as well as SCFA production in healthy rats. In colitic rats, SC-FOS feeding caused a decrease of MPO activity, leukotriene B4 (LTB4) production and iNOS expression. This anti-inflammatory effect was evidenced macroscopically by a significant reduction in the extent of colonic damage. SC-FOS also promoted a more favorable intestinal microbiota, increasing lactobacilli and bifidobacteria counts.
In conclusion, although oligosaccharides are preferentially fermented in the upper parts of the large intestine, its prebiotic effect is extended to the distal colonic segments, thus exerting a positive effect on colonic inflammation.


Similar content being viewed by others
Abbreviations
- DSS::
-
dextran sodium sulfate
- FOS::
-
Fructooligosaccharides
- GF::
-
β-d-fructofuranosyl-(2,1)-α-d-gluco-pyranose
- GF2::
-
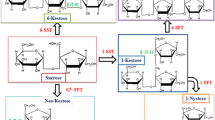
Kestose (β-d-fructofuranosyl-(2,1)-β-d-fructofuranosyl-(2,1)-α-d-gluco-pyranose)
- GF3::
-
Nystose (β-d-fructofuranosyl-(2,1)-β-d-fructofuranosyl-(2,1)-β-d-fructo-furanosyl-(2,1)-α-d-glucopyranose)
- GF4::
-
Fructosyl-Nystose (β-d-fructofuranosyl-(2,1)-β-d-fructofuranosyl-(2,1)-β-d-fructofuranosyl-(2,1)-β-d-fructo-furanosyl-(2,1)-α-d-glucopyranose)
- IBD::
-
inflammatory bowel disease.
- LTB4::
-
leukotriene B4.
- MPO::
-
myeloperoxidase.
- SCFA::
-
short-chain fatty acids.
- SC-FOS::
-
short-chain fructooligosaccharides
- SEM::
-
standard error medium
- TNBS::
-
trinitrobenzenesulfonic acid
- TNF-α::
-
tumor necosis factor-α
References
Macpherson A, Khoo UY, Philipott-Howard J, Bjarnason I (1996) Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38:365–375
Onderdonk AB, Barlett MD (1979) Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr 32:258–265
Sadlack B, Merz H, Schorle H (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 175:253–261
Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Díaz-Ropero MP, Olivares M, Xaus J, Zarzuelo A, Galvez J (2005) Preventive effects of a probiotic, Lactobacillus salivarius ssp. Salivarius, in the TNBS model of rat colitis. World J Gastroenterol 7:11(33):5185–5192
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB (2005) VSL (3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100(7):1539–1546
Videla S, Vilaseca J, Antolin M, Garcia-Lafuente A, Guarner F, Crespo E, Casalots J, Salas A, Malagelada JR (2001) Dietary inulin improves distal colitis induced by dextran sodium sulphate in the rat. Am J Gastroenterol 96(5):1486–1493
Rodriguez-Cabezas ME, Galvez J, Lorente MD, et al. (2002) Dietary fiber down-regulates colonic tumor necrosis factor α in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr 132:3263–3271
Cherbut C, Michel C, Lecannu G (2003) The prebiotic characteristics of Fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J Nutr 133:21–27
Lindsay JO, Whelan K, Stagg AJ, Gobin P, Omar Al-Hassi H, Rayment N, Kamm M, Knight SC, Forbes A (2005) The clinical, microbiological, immunological effects of fructo-oligosaccharides in patients with Crohn’s disease. Gut 14 (Epub ahead of print)
van der Meulen R, Avonts L, de Vuyst L (2004) Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl Environ Microbiol 70(4):1923–1930
Morris GP, Beck PL, Herridge MS, Depew WT, Szewezuk MR, Wallace JL (1989) Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795–803
Mikkelsen LL, Bach Knudsen KE, Jensen BB (2004) In vitro fermentation of fructo-oligosaccharides and trans-galactooligosaccharides by adapted and unadapted bacterial populations from the gastrointestinal tract of piglets. Anim Feed Sci Tech 116:225–238
Bañuelos O, Ronchel MC, Adrio JL, Velasco J. Screening of microorganisms for enzymatic biosynthesis of nondigestible oligosaccharides. In: Barredo JL (ed) Methods in Biotechnology. Vol 17: Microbial Enzymes and Biotransformations. Humana Press Inc. Tokowa NJ
Bell CJ, Gall DG, Wallace JL (1995) Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol 268:G622–G630
Stucchi AF, Shofer S, Leeman S, Materne O, Beer E, McClung J, Shebani K, Moore F, O’Brien M, Becker JM (2000) NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol 279:G1298–G1306
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87:1344–1350
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555
Sánchez de Medina F, Galvez J, Romero JA, Zarzuelo A (1996) Effect of quercitrin on acute and chronic experimental colitis in the rat. J Pharmacol Exp Ther 278:771–779
Camuesco D, Comalada M, Rodríguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Gálvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol 2004. DOI 10.1038/sj.bjp.0705941
Hidaka H, Hirayama M, Tokunaga T, Eida T (1990) The effects of undigestible fructooligosaccharides on intestinal microflora and various physiological functions of human health. Adv Exp Med Biol 270:105–117
Moreau NM, Martin LJ, Toquet CS, Laboisse CL, Nguyen PG, Siliart BS, Dumon HJ, Camp MM (2003) Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by FOS, in DSS-induced experimental colitis. Br J Nutr 90(1):75–85
Kaplan H, Hutkins RW (2000) Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol 66:2682–2684
Kaplan H, Hutkins RW (2003) Metabolism of fructooligosaccharides by Lactobacillus paracasei 1195. Appl Environ Microbiol 69:2217–2222
Houdijk JGM, Bosch MW, Tamminga S, Verstegen MWA, Berenpas EB, Knoop H (1999) Apparent ileal and total-tract nutrient digestion by pigs as affected by dietary nondigestible oligosaccharides. J Anim Sci 77:148–158
Campbell JM, Fahey GC, Bryan WW (1997) Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127:130–136
Zdunczyk Z, Krol B, Juskiewicz J, Wroblewska M (2005) Biological properties of FOS with different contents of kestose and nystose in rats. Arch Anim Nutr 59(4):247–56
Schultz M, Sartor RB (2000) Probiotics and inflammatory bowel diseases. Am J Gastroenterol 95:S19–S21
Acknowledgments
The authors are grateful to Ana Nieto from Health and Progress Foundation (Granada, Spain) and Angel Concha from the hospital ‘Virgen de las Nieves’ (Granada, Spain) for their kind help in the microscopic histological analysis. We would also like to thank Dr. Arjan Geerlings for the English revision of the manuscript. Monica Comalada is a recipient of a grant from the Juan de la Cierva Program of Spanish Ministry of Science and Technology. Desirée Camuesco is a recipient of a grant form the Junta de Andalucía (Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work has been supported by Puleva Biotech own founds and by Instituto de Salud Carlos III (PI0121732) with funds from the European Union and Junta de Andalucía (CTS 164).
An erratum to this article is available athttp://dx.doi.org/10.1007/s00394-006-0615-x.
Rights and permissions
About this article
Cite this article
Lara-Villoslada, F., de Haro, O., Camuesco, D. et al. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. Eur J Nutr 45, 418–425 (2006). https://doi.org/10.1007/s00394-006-0610-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-006-0610-2