Abstract
Key message
This is the first report assessing epigenetic variation in garlic. High genetic and epigenetic polymorphism during in vitro culture was detected. Sequencing of MSAP fragments revealed homology with ESTs.
Abstract
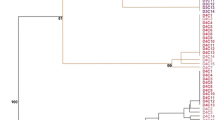
Garlic (Allium sativum) is a worldwide crop of economic importance susceptible to viral infections that can cause significant yield losses. Meristem tissue culture is the most employed method to sanitize elite cultivars. Often the virus-free garlic plants obtained are multiplied in vitro (micro propagation). However, it was reported that micro-propagation frequently produces somaclonal variation at the phenotypic level, which is an undesirable trait when breeders are seeking to maintain varietal stability. We employed amplification fragment length polymorphism and methylation sensitive amplified polymorphism (MSAP) methodologies to assess genetic and epigenetic modifications in two culture systems: virus-free plants obtained by meristem culture followed by in vitro multiplication and field culture. Our results suggest that garlic exhibits genetic and epigenetic polymorphism under field growing conditions. However, during in vitro culture system both kinds of polymorphisms intensify indicating that this system induces somaclonal variation. Furthermore, while genetic changes accumulated along the time of in vitro culture, epigenetic polymorphism reached the major variation at 6 months and then stabilize, being demethylation and CG methylation the principal conversions. Cloning and sequencing differentially methylated MSAP fragments allowed us to identify coding and unknown sequences of A. sativum, including sequences belonging to LTR Gypsy retrotransposons. Together, our results highlight that main changes occur in the initial 6 months of micro propagation. For the best of our knowledge, this is the first report on epigenetic assessment in garlic.


Similar content being viewed by others
References
Al-Zahim MA, Ford-Lloyd BV, Newbury HJ (1999) Detection of somaclonal variation in garlic (Allium sativum L.) using RAPD and cytological analysis. Plant Cell Rep 18:473–477. doi:10.1007/s002990050606
Amir R, Hacham Y, Galili G (2002) Cystathionine γ-synthase and threonine synthase operate in concert to regulate carbon flow towards methionine in plants. Trends Plant Sci 7:153156. doi:10.1016/S1360-1385(02)02227-6
Baránek M, Křižan B, Ondrušíková E, Pidra M (2010) DNA-methylation changes in grapevine somaclones following in vitro culture and thermotherapy. Plant Cell Tissue Organ Cult 101:11–22. doi:10.1007/s11240-009-9656-1
Bobadilla Landey R, Cenci A, Georget F et al (2013) High genetic and epigenetic stability in Coffea arabica plants derived from embryogenic suspensions and secondary embryogenesis as revealed by AFLP, MSAP and the phenotypic variation rate. PLoS One 8:e56372. doi:10.1371/journal.pone.0056372
Brenchley R, Spannagl M, Pfeifer M et al (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491:705–710. doi:10.1038/nature11650
Burba JL (2003) Producción de ajo, Instituto Nacional de Tecnología Agropecuaria. La Consulta, Mendoza, Argentina
Carvalho MG (1981) Viroses do alho. Fitopatologia Brasileira 6:299–300
Carvalho MG, Shepherd RR, Hall DH (1981) Virus em clone de alho sem sintomas e liberto do Garlic yellow stripe virus. Fitopatologia Brasileira 6:236
Chandler VL, Walbot V (1986) DNA modification of a maize transposable element correlates with loss of activity. Proc Natl Acad Sci USA 83:1767–1771. doi:10.1073/pnas.83.6.1767
Conci VC (1997) An overview of Allium viruses in Argentina. Acta Hortic 433:593–600
Conci VC, Nome S (1991) Virus free garlic (Allium sativum L.) plants obtained by thermotherapy and meristem tip culture. J Phytopathol 132:186–192
Conci VC, Moriconi DN, Nome SF (1986) Cultivo de meristemas apicales de seis diferentes tipos clonales de ajo (Allium sativum L.). Phyton 46:187–194
Conci VC, Helguera M, Nome SF (1999) Serological and biological comparison of Onion yellow dwarf virus from onion and garlic in Argentina. Fitopatologia Brasilera 24:73–75
Conci VC, Canavelli A, Lunello P, Di Rienzo J, Nome SF, Zumelzu G, Italia R (2003) Yield losses associated with virus-infected garlic plants during five successive years. Plant Dis 87:1411–1415
Conci VC, Perotto MC, Cafrune E, Lunello P (2005) Program for intensive production of virus-free garlic plants. Acta Hortic 688:195–200
Conci VC, Canavelli A, Balzarini M (2010) The distribution of garlic viruses in leaves and bulbs during the first year of infection. J Phytopathol 158:186–193. doi:10.1111/j.1439-0434.2009.01601.x
Dann AL, Wilson CR (2011) Comparative assessment of genetic and epigenetic variation among regenerants of potato (Solanum tuberosum) derived from long-term nodal tissue-culture and cell selection. Plant Cell Rep 30:631–639. doi:10.1007/s00299-010-0983-9
De-la-Peña C, Nic-Can G, Ojeda G et al (2012) KNOX1 is expressed and epigenetically regulated during in vitro conditions in Agave spp. BMC Plant Biol 12:203. doi:10.1186/1471-2229-12-203
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2014) InfoStat versión 2014. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Díaz-Martínez M, Nava-Cedillo A, Guzmán-López JA et al (2012) Polymorphism and methylation patterns in Agave tequilana Weber var. “Azul” plants propagated asexually by three different methods. Plant Sci 185–186:321–330. doi:10.1016/j.plantsci.2012.01.004
Douet, Tourmente (2007) Transcription of the 5S rRNA heterochromatic genes is epigenetically controlled in Arabidopsis thaliana and Xenopus laevis. Heredity 99:5–13. doi:10.1038/sj.hdy.6800964
Ellis TH, Lee D, Thomas CM, Simpson PR, Cleary WG, Newman MA et al (1988) 5S rRNA genes in Pisum: sequence, long range and chromosomal organization. Mol Gen Genet 214:333–342
FAO (2014) Proposal for new work on a codex standard for garlic (Codex Alimentarius Comission, CX/FFV 14/18/10 - ADD.1; January 2014). ftp://ftp.fao.org/codex/meetings/ccffv/ccffv18/ff18_10_add1e.pdf. Accessed 10 Sept 2015
Fedoroff NV (2012) Transposable elements, epigenetics, and genome evolution. Science 80(338):758–767. doi:10.1126/science.338.6108.758
Fraga HPF, Vieira LN, Caprestano CA et al (2012) 5-Azacytidine combined with 2,4-D improves somatic embryogenesis of Acca sellowiana (O. Berg) Burret by means of changes in global DNA methylation levels. Plant Cell Rep 31:2165–2176. doi:10.1007/s00299-012-1327-8
Fulnecek J, Matyasek R, Kovarik A, Bezdek M (1998) Mapping of 5-methylcytosine residues in Nicotiana tabacum 5S rRNA genes by genomic sequencing. Mol Gen Genet 259:133–141
Gao X, Yang D, Cao D et al (2009) In vitro micro propagation of Freesia hybrida and the assessment of genetic and epigenetic stability in regenerated plantlets. J Plant Growth Regul 29:257–267. doi:10.1007/s00344-009-9133-4
Garcia S, Khaitová L, Kovarík A (2012) Expression of 5S rRNA genes linked to 35S rDNA in plants, their epigenetic modification and regulatory element divergence. BMC Plant Biol 12:95. doi:10.1186/1471-2229-12-95
Goldsbrough PB, Ellis TH, Lomonossoff GP (1982) Sequence variation and methylation of the flax 5S RNA genes. Nucleic Acids Res 10:4501–4514
Grellet F, Penon P (1984) Chromatin organization and methylation patterns of wheat 5S RNA genes. Plant Sci Lett 37:129–136
Helguera MP, Lunello P, Nome C, Conci VC (1997) Advances in the purification of filamentous viruses from garlic and in antisera production. Acta Hortic 433:623–630
Huang H, Han SS, Wang Y et al (2012) Variations in leaf morphology and DNA methylation following in vitro culture of Malus xiaojinensis. Plant Cell Tissue Organ Cult 111:153–161. doi:10.1007/s11240-012-0179-9
Iciek M, Kwiecień I, Włodek L (2009) Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen 50:247–265. doi:10.1002/em.20474
Jakše J, Meyer JDF, Suzuki G et al (2008) Pilot sequencing of onion genomic DNA reveals fragments of transposable elements, low gene densities, and significant gene enrichment after methyl filtration. Mol Genet Genomics 280:287–292. doi:10.1007/s00438-008-0364-z
Kaeppler SM, Phillips RL (1993) Tissue culture-induced DNA methylation variation in maize. Proc Natl Acad Sci USA 90:8773–8776. doi:10.1073/pnas.90.19.8773
Kaeppler SM, Parke JL, Mueller SM et al (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40:358–364
Kashkush K, Yaakov B (2011) Methylation, transcription, and rearrangements of transposable elements in synthetic allopolyploids. Int J Plant Genomics. doi:10.1155/2011/569826
Kim DW, Jung TS, Nam SH et al (2009) Garlic ESTdb: an online database and mining tool for garlic EST sequences. BMC Plant Biol 9:61. doi:10.1186/1471-2229-9-61
Kotchoni SO, Gachomo EW, Betiku E, Olusoji O (2003) A home made kit for plasmid DNA mini-preparation. Afr J Biotechnol 2:88–90
Krzymińska A, Gawłowska M, Wolko B, Bocianowski J (2008) Genetic diversity of ornamental Allium species and cultivars assessed with isozymes. J Appl Genet 49:213–220. doi:10.1007/BF03195616
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214. doi:10.1007/BF02342540
Li X, Yu X, Wang N et al (2007) Genetic and epigenetic instabilities induced by tissue culture in wild barley (Hordeum brevisubulatum (Trin.) Link). Plant Cell Tissue Organ Cult 90:153–168. doi:10.1007/s11240-007-9224-5
Lunello P, Ducasse D, Helguera M, Nome SF, Conci VC (2002) An Argentinean isolate of Leek yellow tripe virus from leek can be transmitted to garlic. J Plant Pathol 84:11–17
Lunello P, Di Rienzo J, Conci VC (2007) Yield loss in garlic caused by leek yellow stripe virus Argentinean isolate. Plant Dis 91:153–158. doi:10.1094/PDIS-91-2-0153
Mascia PN, Rubenstein I, Phillips RL, Wang AS, Xiang LZ (1981) Localization of the 5S rRNA genes and evidence for diversity in the 5S rDNA region of maize. Gene 15:7–20
McCallum J, Baldwin S, Shigyo M et al (2012) AlliumMap-A comparative genomics resource for cultivated Allium vegetables. BMC Genom 13:168. doi:10.1186/1471-2164-13-168
Milne RG, Luisoni E (1977) Rapid immune electron microscopy of virus preparations. In: Maramorosch K, Koprowski H (eds) Methods in Virology, vol 6. Academic Press, New York, pp 265–281
Moriconi DN, Conci VC, Nome SF (1990) Rapid multiplication of garlic (Allium sativum L.) in vitro. Phyton 51:145–151
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326. doi:10.1093/nar/8.19.4321
Ngezahayo F, Xu C, Wang H et al (2009) Tissue culture-induced transpositional activity of mPing is correlated with cytosine methylation in rice. BMC Plant Biol 9:91. doi:10.1186/1471-2229-9-91
Paterson AH, Bowers JE, Bruggmann R et al (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556. doi:10.1038/nature07723
Paz RC, Rendina González AP, Ferrer MS, Masuelli RW (2015) Short-term hybridization activates Tnt1 and Tto1 Copia retrotransposons in wild tuber-bearing Solanum species. Plant Biol 17(4):860–869. doi:10.1111/plb.12301
Peredo EL, Arroyo-García R, Revilla MÁ (2009) Epigenetic changes detected in micropropagated hop plants. J Plant Physiol 166:1101–1111. doi:10.1016/j.jplph.2008.12.015
Perotto MC, Conci VC, Cafrune EE, Alochis P, Bracamonte R (2003) Differences in the response of garlic cultivars to the eradication of five viruses. Phyton Int J Exp Bot: 233–240
Perotto MC, Cafrune EE, Conci VC (2010) The effect of additional viral infections on garlic plants initially infected with Allexiviruses. Eur J Plant Pathol 126:489–495. doi:10.1007/s10658-009-9555-7
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91:5222–5226
Piccolo FM, Fisher AG (2014) Getting rid of DNA methylation. Trends Cell Biol 24:136–143. doi:10.1016/j.tcb.2013.09.001
Pittler MH, Ernst E (2007) Clinical effectiveness of garlic (Allium sativum). Mol Nutr Food Res 51:1382–1385. doi:10.1002/mnfr.200700073
Rafalski JA, Wiewiorowski M, Soll D (1982) Organization and nucleotide sequence of nuclear 5S rRNA genes in yellow lupin (Lupinus luteus). Nucleic Acids Res 10:7635–7642
Ramírez-Malagón, Pérez-Moreno, Borodanenko et al (2006) Differential organ infection studies, potyvirus elimination, and field performance of virus-free garlic plants produced by tissue culture. Plant Cell Tissue Organ Cult 86:103–110. doi:10.1007/s11240-006-9102-6
Rival A, Ilbert P, Labeyrie A et al (2013) Variations in genomic DNA methylation during the long-term in vitro proliferation of oil palm embryogenic suspension cultures. Plant Cell Rep 32:359–368. doi:10.1007/s00299-012-1369-y
Rivlin RS (2001) Historical perspective on the use of garlic. J Nutr 131:951–954
Rodríguez López CM, Wetten AC, Wilkinson MJ (2010) Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol 186:856–868. doi:10.1111/j.1469-8137.2010.03242.x
Rohlf FJ (1998) NTSYS-pc: numerical taxonomy system, ver. 2.1. Exeter Publications, Setauket
Santos MDM, Buso GCS, Torres AC (2008) Evaluation of genetic variability in micropropagated propagules of ornamental pineapple [Ananas comosus var. bracteatus (Lindley) Coppens and Leal] using RAPD markers. Genet Mol Res 7:1097–1105. doi:10.4238/vol7-4gmr489
Schlüter PM, Harris SA (2006) Analysis of multilocus fingerprinting data sets containing missing data. Mol Ecol Notes 6:569–572. doi:10.1111/j.1471-8286.2006.01225.x
Shahraeen N, Lesemann DE, Ghotbi T (2008) Survey for viruses infecting onion, garlic and leek crops in Iran. EPPO Bull 38:131–135. doi:10.1111/j.1365-2338.2008.01198.x
Simon PW, Jenderek MM (2003) Flowering, seed production and the genesis of garlic breeding. 23:211–244
Smulders MJM, Rus-Kortekaas W, Vosman B (1995) Tissue culture-induced DNA methylation polymorphisms in repetitive DNA of tomato calli and regenerated plants. Theor Appl Genet 91:1257–1264. doi:10.1007/BF00220938
Sun X, Zhou S, Meng F, Liu S (2012) De novo assembly and characterization of the garlic (Allium sativum) bud transcriptome by Illumina sequencing. Plant Cell Rep 31:1823–1828. doi:10.1007/s00299-012-1295-z
Sun S, Zhong J, Li S, Wang X (2013) Tissue culture-induced somaclonal variation of decreased pollen viability in torenia (Torenia fournieri Lind.). Bot Stud 54:36. doi:10.1186/1999-3110-54-36
Suzuki G, Ura A, Saito N et al (2001) BAC FISH analysis in Allium cepa. Genes Genet Syst 76:251–255. doi:10.1266/ggs.76.251
Taşkin H, Baktemur G, Kurul M, Büyükalaca S (2013) Use of tissue culture techniques for producing virus-free plant in garlic and their identification through real-time PCR. Sci World J. doi:10.1155/2013/781282
Torres-Morán MI, Escoto-Delgadillo M, Molina-Moret S et al (2010) Assessment of genetic fidelity among Agave tequilana plants propagated asexually via rhizomes versus in vitro culture. Plant Cell Tissue Organ Cult 103:403–409. doi:10.1007/s11240-010-9777-6
Us-Camas R, Rivera-Solís G, Duarte-Aké F, De-la-Peña C (2014) In vitro culture: an epigenetic challenge for plants. Plant Cell Tissue Organ Cult 118:187–201. doi:10.1007/s11240-014-0482-8
Vitte C, Estep MC, Leebens-Mack J, Bennetzen JL (2013) Young, intact and nested retrotransposons are abundant in the onion and asparagus genomes. Ann Bot 112:881–889. doi:10.1093/aob/mct155
Volk GM, Henk AD, Richards CM (2004) Genetic diversity among US garlic clones as detected using AFLP methods. J Am Soc Hortic Sci 129:559–569
Vos P, Hogers R, Bleeker M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414. doi:10.1093/nar/23.21.4407
Wang QM, Wang YZ, Sun LL et al (2012) Direct and indirect organogenesis of Clivia miniata and assessment of DNA methylation changes in various regenerated plantlets. Plant Cell Rep 31:1283–1296. doi:10.1007/s00299-012-1248-6
Wang X, Wu R, Lin X et al (2013) Tissue culture-induced genetic and epigenetic alterations in rice pure-lines, F1 hybrids and polyploids. BMC Plant Biol 13:77. doi:10.1186/1471-2229-13-77
Wu Y, Wu R, Zhang B et al (2011) Epigenetic instability in genetically stable micro propagated plants of Gardenia jasminoides Ellis. Plant Growth Regul 66:137–143. doi:10.1007/s10725-011-9637-3
Xiong LZ, Xu CG, Saghai Maroof MA, Zhang Q (1999) Patterns of cytosine methylation in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Mol Gen Genet 261:439–446. doi:10.1007/s004380050986
Xu M, Li X, Korban SS (2004) DNA-methylation alterations and exchanges during in vitro cellular differentiation in rose (Rosa hybrida L.). Theor Appl Genet 109:899–910. doi:10.1007/s00122-004-1717-6
Yap I, Nelson RJ (1996) WinBoot: a program for performing bootstrap analysis of binary data to determine the confidence limits of UPGMA-based dendrograms. International Rice Research Institute, Manila
Yu J, Hu S, Wang J et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296:79–92. doi:10.1126/science.1068037
Acknowledgments
The authors would like to thank M.Sc. José Luis Burba for kindly supplying vegetal materials and making suggestions, Dr. Marcos Celli for its collaboration in plant virus analysis, Dr. Ricardo W. Masuelli for generously providing equipment and for his valuable comments, to Dr. Nicolás Cara for technical support and to PhD María Virginia Sánchez Puerta for her careful revision of this manuscript and valuable comments. The present work was funded by grants from INTA (National Institute for Agricultural Technology) through the project “Generación de tecnologías alternativas frente a los nuevos escenarios para la sustentabilidad de la cadena de valor de ajo diferenciado” (INTA-PNHFA061251) and by the grants from SECTyP (Science, Technique and Postgraduate Secretariat) through the project “Estrés y cambios asociados a la metilación de ADN en plantas de ajo (Allium sativum L.) cultivadas in vitro: influencia del genotipo (Parte II)” (SECTyP UNCuyo 06/A49).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Communicated by Z.-Y. Wang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2015_1874_MOESM4_ESM.tif
Suppl. Figure 1 Diagram of vegetal material showing the two culture systems used in this work: field culture and meristem regeneration followed by in vitro propagation. The original material, Perla INTA accession was cultured in soil and in vitro by meristem plus micro propagation culture. Three linages of virus-free plants were selected. For DNA extraction leaf samples from three replicates were excised (FA, FB, FC) from meristem field-grown plants (MA, MB, MC), 6 (IV6A, IV6B, IV6C) and 12 (IV12A, IV12B, IV12C) months micropropagated plants in vitro. (TIFF 6750 kb)
Rights and permissions
About this article
Cite this article
Gimenez, M.D., Yañez-Santos, A.M., Paz, R.C. et al. Assessment of genetic and epigenetic changes in virus-free garlic (Allium sativum L.) plants obtained by meristem culture followed by in vitro propagation. Plant Cell Rep 35, 129–141 (2016). https://doi.org/10.1007/s00299-015-1874-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1874-x