Abstract
Background
R1507 is a human IgG1 Mab that binds to the insulin-like growth factor-1 receptor (IGF-1R) and inhibits IGF-1- or IGF-2-mediated anchorage-independent growth of malignant cells. A phase 1b study evaluated the safety, tolerability and efficacy of R1507 in combination with multiple standard oncology regimens.
Methods
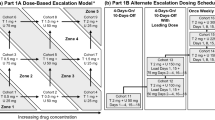
R1507 (3, 5, 9, 10 and 16 mg/kg IV, Q2 W or Q3 W) was added to six treatment regimens: gemcitabine + erlotinib (GE); paclitaxel + bevacizumab (PB); carboplatin + etoposide (CE); mFOLFOX6 + bevacizumab (FB); capecitabine + trastuzumab (CT); and sorafenib (S). If tolerable, R1507 dose was escalated utilizing a 3 + 3 + 6 and a 3 + 9 design.
Results
A total of 104 patients enrolled into regimens 1–6: 93 % were non-recent diagnoses. Eighteen withdrew for safety [one death, 17 adverse events (AEs)]. A total of 1,337 AEs any grade, across regimens and doses were nausea, vomiting and diarrhea. A total of 123 had grade ≥3 AEs (n = 28 dose level 1; n = 95 dose level 1) and in 60 patients were myelosuppression, fatigue and mucosal inflammation. ORR (PR plus SD) of evaluable patients across six regimens was 36 % with five PRs: regimens PB (non-small cell lung cancer, nasopharyngeal cancer), CE (melanoma), FB (colon cancer) and S (GIST). The GIST pt (>4 prior therapies) had a PR for 3 years. Three patients (breast cancer, melanoma and adenoid cystic carcinoma) were on study for >1 year; 76 % of patients had SD or better for 4 months.
Conclusions
R1507 added to six standard oncology regimens was well tolerated with an ORR of 36 %.


Similar content being viewed by others
References
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4(7):505–518
Baserga R (2005) The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opin Ther Targets 9(4):753–768
Kurmasheva RT, Houghton PJ (2006) IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta 1766(1):1–22
Sachdev D, Yee D (2007) Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 6(1):1–12
Tao Y, Pinzi V, Bourhis J, Deutsch E (2007) Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway–therapeutic perspectives in cancer. Nat Clin Pract Oncol 4(10):591–602
Stattin P, Bylund A, Rinaldi S et al (2000) Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst 92:1910–1917
Rosen N, Yee D, Lippman ME et al (1991) Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat 18(suppl 1):S55–S62
Angelloz-Nicoud P, Binoux M (1995) Autocrine regulation of cell proliferation by the insulin-like growth factor (IGF) and IGF binding protein-3 protease system in a human prostate carcinoma cell line (PC-3). Endocrinology 136:5485–5492
Rotsch M, Maasberg M, Erbil C et al (1992) Characterization of insulin-like growth factor 1 receptors and growth effects in human lung cancer cell lines. J Cancer Res Clin Oncol 118:502–508
Culouscou JM, Shoyab M (1991) Purification of a colon cancer cell growth inhibitor and its identification as an insulin-like growth factor binding protein. Cancer Res 51:2813–2819
Sekyi-Otu A, Bell RS, Ohashi C et al (1995) Insulin-like growth factor 1 (IGF-1) receptors, IGF-1, and IGF-2 are expressed in primary human sarcomas. Cancer Res 55:129–134
Bergmann U, Funatomi H, Kornmann M et al (1996) Increased expression of insulin receptor substrate-1 in human pancreatic cancer. Biochem Biophys Res Commun 220:886–890
Goetsch L, Gonzalez A, Leger O et al (2005) A recombinant humanized anti- insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer 113(2):316–328
Lu D, Zhang H, Koo H et al (2005) A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem 280(20):19665–19672
Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY (2007) Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res 13(9):2795–2803
Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY (2006) Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res 66(20):10100–10111
Karp, D et al (2007) Efficacy of the anti-insulin like growth factor I receptor (IGF- 1R) antibody CP 751871 in combination with paclitaxel and carboplatin as first-line treatment for advanced non-small cell lung cancer (NSCLC). J Clin Oncol, ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 7506
Karp, D (2008) High Activity of the anti-IGFR antibody CP-751,871 in combination with paclitaxel and carboplatin in squamous cell carcinoma. J Clin Oncol 26: 2008 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (May 20 suppl; abstr 8015)
Sarantopoulos, J et al (2008) A phase 1B study of AMG 479, a type 1 insulin-like growth factor receptor (IGF-1R) antibody, in combination with panitumumab(P) or gemcitabine (G). J Clin Oncol 26: 2008 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (May 20 suppl; abstr 3583)
Razelle Kurzrock, Amita Patnaik, Joseph Aisner et al (2010) A Phase I Study of Weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist in patients with solid tumors. Clin Cancer Resm 16:2458–2465
Von Hoff DD, Nieves JA, Vocila LK, Weitman SD, Cvitkovic EC (2007) The complete phase Ib clinical trial: A method to accelerate new agent development. J Clin Oncol 25(18S 2007 ASCO Annual Meeting Proceedings Part I, abstract 2562)
National Cancer Institute (2006) CTC v2.0 and Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda, MD: National Cancer Institute, 2006. Available at http://ctep.info.nih.gov/reporting/ctc.html
Nannini M, Astolfi A, Paterini P, Urbini M, Santini D, Catena F, Indio V, Casadio R, Pinna AD, Biasco G, Pantaleo MA (2013) Expression of IGF-1 receptor in KIT/PDGF receptor-α wild-type gastrointestinal stromal tumors with succinate dehydrogenase complex dysfunction. Future Oncol 9(1):121–126. doi:10.2217/fon.12.170
Pappo AS, Patel SR, Crowley J et al (2011) R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II sarcoma alliance for research through collaboration study. J Clin Oncol 29:4541–4547
Jassem J, Langer CJ, Karp DD et al (2010) Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol; 28 (suppl): abstr 7500
Robertson JFR, Ferrero J-M, Bourgeois H et al (2013) Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol; published online Feb 13
Di Cosimo S, Scaltriti M, Val D et al (2007) The PI3-K/AKT/mTOR pathway as a target for breast cancer therapy. Proc Am Soc Clin Oncol. Abstr 3511
Acknowledgments
We wish to thank Roche Pharmaceuticals for supporting this trial, clinical research coordinators, research nurses and patients and their families for participating in this trial. We wish to thank Elizabeth Claire Dees, M.D., University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA and Michael L. Maitland, M.D., University of Chicago Medicine, Chicago, IL, USA for their participation in this trial.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahadevan, D., Sutton, G.R., Arteta-Bulos, R. et al. Phase 1b study of safety, tolerability and efficacy of R1507, a monoclonal antibody to IGF-1R in combination with multiple standard oncology regimens in patients with advanced solid malignancies. Cancer Chemother Pharmacol 73, 467–473 (2014). https://doi.org/10.1007/s00280-013-2372-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2372-x