Abstract
Purpose
To assess the potential for drug–drug interactions between lenalidomide and substrates and inhibitors of cytochrome P450 (CYP) isozymes.
Methods
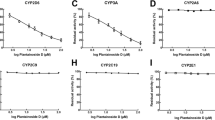
In vitro metabolism of lenalidomide by human liver microsomes, recombinant human CYPs and human hepatocytes was evaluated. The inhibitory and inductive effects of lenalidomide on the CYP activities were evaluated in human liver microsomes and cultured human hepatocytes, respectively.
Results
In vitro incubation of lenalidomide with human liver microsomes, recombinant-CYP isozymes, and human hepatocytes did not result in Phase I or Phase II metabolism, confirming the low propensity of lenalidomide for metabolism in vivo in humans. In vitro, lenalidomide did not inhibit CYP isozymes in human liver microsomes and did not induce CYP activities in cultured human hepatocytes.
Conclusions
Lenalidomide is not a substrate, inhibitor, or inducer of CYP group of enzymes; clinically relevant pharmacokinetic drug–drug interactions are unlikely to occur between lenalidomide and co-administered CYP substrates or inhibitors.
Similar content being viewed by others
References
Schafer PH, Gandhi AK, Loveland MA et al (2003) Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther 305(3):1222–1232
Gandhi AK, Kang J, Naziruddin S et al (2006) Lenalidomide inhibits proliferation of Namalwa CSN. 70 cells and interferes with Gab1 phosphorylation and adaptor protein complex assembly. Leuk Res 30(7):849–858
List A, Kurtin S, Roe DJ et al (2005) Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 352(6):549–557
Melchert M, Kale V, List A (2007) The role of lenalidomide in the treatment of patients with chromosome 5q deletion and other myelodysplastic syndromes. Curr Opin Hematol 14(2):123–129
Richardson PG, Blood E, Mitsiades CS et al (2006) A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood 108(10):3458–3464
Rajkumar SV, Hayman SR, Lacy MQ et al (2005) Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood 106(13):4050–4053
Thomas S, Alexanian R (2007) Current treatment strategies for multiple myeloma. Clin Lymphoma Myeloma 7(suppl 4):S139–S144
Molica S (2007) Immunomodulatory drugs in chronic lymphocytic leukemia: a new treatment paradigm. Leuk Lymphoma 48(5):866–869
Dreicer R (2007) Lenalidomide: immunomodulatory, antiangiogenic, and clinical activity in solid tumors. Curr Oncol Rep 9(2):120–123
Chanan-Khan AA, Cheson BD (2008) Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol 26(9):1544–1552
Back DJ, Orme ML (1990) Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet 18(6):472–484
Park BK, Kitteringham NR, Pirmohamed M et al (1996) Relevance of induction of human drug-metabolizing enzymes: pharmacological and toxicological implications. Br J Clin Pharmacol 41(6):477–491
Gentile DM, Tomlinson ES, Maggs JL et al (1996) Dexamethasone metabolism by human liver in vitro. Metabolite identification and inhibition of 6-hydroxylation. J Pharmacol Exp Ther 277(1):105–112
Pascussi JM, Drocourt L, Fabre JM et al (2000) Dexamethasone induces pregnane X receptor and retinoid X receptor—alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 58(2):361–372
LeCluyse E, Madan A, Hamilton G et al (2000) Expression and regulation of cytochrome P450 enzymes in primary cultures of human hepatocytes. J Biochem Mol Toxicol 14(4):177–188
Madan A, Graham RA, Carroll KM et al (2003) Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos 31(4):421–431
Quistorff B, Dich J, Grunnet N (1989) Preparation of isolated rat liver hepatocytes. In: Pollard JW, Walker JM (eds) Methods in molecular biology, vol 5. Animal cell culture. Humana Press, New Jersey, pp 151–160
Chen N, Lau H, Kong L et al (2007) Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol 47(12):1466–1475
Acknowledgments
Authors would like to thank Rebecca R. Campbell of Xenotech LLC, and Anthony Glazer of Covance Laboratories Inc., for their contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, G., Lau, H. & Laskin, O. Lenalidomide: in vitro evaluation of the metabolism and assessment of cytochrome P450 inhibition and induction. Cancer Chemother Pharmacol 63, 1171–1175 (2009). https://doi.org/10.1007/s00280-008-0867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0867-7