Abstract
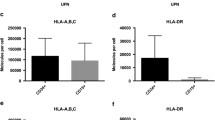
The identification and validation of new cancer-specific T cell epitopes continues to be a major area of research interest. Nevertheless, challenges remain to develop strategies that can easily discover and validate epitopes expressed in primary cancer cells. Regarded as targets for T cells, peptides presented in the context of the major histocompatibility complex (MHC) are recognized by monoclonal antibodies (mAbs). These mAbs are of special importance as they lend themselves to the detection of epitopes expressed in primary tumor cells. Here, we use an approach that has been successfully utilized in two different infectious disease applications (WNV and influenza). A direct peptide-epitope discovery strategy involving mass spectrometric analysis led to the identification of peptide YLLPAIVHI in the context of MHC A*02 allele (YLL/A2) from human breast carcinoma cell lines. We then generated and characterized an anti-YLL/A2 mAb designated as RL6A TCRm. Subsequently, the TCRm mAb was used to directly validate YLL/A2 epitope expression in human breast cancer tissue, but not in normal control breast tissue. Moreover, mice implanted with human breast cancer cells grew tumors, yet when treated with RL6A TCRm showed a marked reduction in tumor size. These data demonstrate for the first time a coordinated direct discovery and validation strategy that identified a peptide/MHC complex on primary tumor cells for antibody targeting and provide a novel approach to cancer immunotherapy.





Similar content being viewed by others
References
Weiner LM, Belldegrun AS, Crawford J, Tolcher AW, Lockbaum P, Arends RH, Navale L, Amado RG, Schwab G, Figlin RA (2008) Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res 14:502–508
Halama N, Herrmann C, Jaeger D, Herrmann T (2008) Treatment with cetuximab, bevacizumab and irinotecan in heavily pretreated patients with metastasized colorectal cancer. Anticancer Res 28:4111–4115
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334
Kabbinavar F, Irl C, Zurlo A, Hurwitz H (2008) Bevacizumab improves the overall and progression-free survival of patients with metastatic colorectal cancer treated with 5-fluorouracil-based regimens irrespective of baseline risk. Oncology 75:215–223
Cartwright T, Kuefler P, Cohn A, Hyman W, Berger M, Richards D, Vukelja S, Nugent JE, Ruxer RL Jr, Boehm KA et al (2008) Results of a phase II trial of cetuximab plus capecitabine/irinotecan as first-line therapy for patients with advanced and/or metastatic colorectal cancer. Clin Colorectal Cancer 7:390–397
Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z (2003) Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2:1113–1120
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J (1998) Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58:2825–2831
Shastri N, Schwab S, Serwold T (2002) Producing nature’s gene-chips: the generation of peptides for display by MHC class I molecules. Annu Rev Immunol 20:463–493
Brodsky FM, Lem L, Bresnahan PA (1996) Antigen processing and presentation. Tissue Antigens 47:464–471
Alexander RB, Brady F, Leffell MS, Tsai V, Celis E (1998) Specific T cell recognition of peptides derived from prostate-specific antigen in patients with prostate cancer. Urology 51:150–157
Wolfel T, Hauer M, Klehmann E, Brichard V, Ackermann B, Knuth A, Boon T, Meyer Zum Buschenfelde KH (1993) Analysis of antigens recognized on human melanoma cells by A2-restricted cytolytic T lymphocytes (CTL). Int J Cancer 55:237–244
Wahl A, Weidanz J, Hildebrand W (2006) Direct class I HLA antigen discovery to distinguish virus-infected and cancerous cells. Expert Rev Proteomics 3:641–652
Kessler JH, Melief CJ (2007) Identification of T-cell epitopes for cancer immunotherapy. Leukemia 21:1859–1874
Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG (2003) IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res 31:311–314
Robinson J, Marsh SG (2003) HLA informatics: accessing HLA sequences from sequence databases. Methods Mol Biol 210:3–21
Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T (2002) Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer 102:499–506
Rosenberg SA (1996) Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst 88:1635–1644
Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH (1992) Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science 255:1261–1263
Hawkins OE, Vangundy RS, Eckerd AM, Bardet W, Buchli R, Weidanz JA, Hildebrand WH (2008) Identification of breast cancer peptide epitopes presented by HLA-A*0201. J Proteome Res 7:1445–1457
Hickman HD, Luis AD, Buchli R, Few SR, Sathiamurthy M, VanGundy RS, Giberson CF, Hildebrand WH (2004) Toward a definition of self: proteomic evaluation of the class I peptide repertoire. J Immunol 172:2944–2952
Prilliman KR, Jackson KW, Lindsey M, Wang J, Crawford D, Hildebrand WH (1999) HLA-B15 peptide ligands are preferentially anchored at their C termini. J Immunol 162:7277–7284
Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S (1996) A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc Natl Acad Sci USA 93:1820–1824
Zhong G, Reis e Sousa C, Germain RN (1997) Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med 186:673–682
Weidanz JA, Nguyen T, Woodburn T, Neethling FA, Chiriva-Internati M, Hildebrand WH, Lustgarten J (2006) Levels of specific peptide–HLA class I complex predicts tumor cell susceptibility to CTL killing. J Immunol 177:5088–5097
Weidanz JA, Piazza P, Hickman-Miller H, Woodburn D, Nguyen T, Wahl A, Neethling F, Chiriva-Internati M, Rinaldo CR, Hildebrand WH (2007) Development and implementation of a direct detection, quantitation and validation system for class I MHC self-peptide epitopes. J Immunol Methods 318:47–58
Prilliman K, Lindsey M, Zuo Y, Jackson KW, Zhang Y, Hildebrand W (1997) Large-scale production of class I bound peptides: assigning a signature to HLA-B*1501. Immunogenetics 45:379–385
Wei ML, Cresswell P (1992) HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature 356:443–446
Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM (1996) Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94–96
Wittman VP, Woodburn D, Nguyen T, Neethling FA, Wright S, Weidanz JA (2006) Antibody targeting to a class I MHC-peptide epitope promotes tumor cell death. J Immunol 177:4187–4195
Novellino L, Castelli C, Parmiani G (2005) A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother 54:187–207
Clark CE, Vonderheide RH (2005) Getting to the surface: a link between tumor antigen discovery and natural presentation of peptide–MHC complexes. Clin Cancer Res 11:5333–5336
Mosca PJ, Hobeika AC, Clay TM, Morse MA, Lyerly HK (2001) Direct detection of cellular immune responses to cancer vaccines. Surgery 129:248–254
Wahl A, Schafer F, Bardet W, Buchli R, Air GM, Hildebrand WH (2009) HLA class I molecules consistently present internal influenza epitopes. Proc Natl Acad Sci USA 106:540–545
Parsons R, Lelic A, Hayes L, Carter A, Marshall L, Evelegh C, Drebot M, Andonova M, McMurtrey C, Hildebrand W et al (2008) The memory T cell response to West Nile virus in symptomatic humans following natural infection is not influenced by age and is dominated by a restricted set of CD8+ T cell epitopes. J Immunol 181:1563–1572
Heinlein UA (1998) Dead box for the living. J Pathol 184:345–347
Endoh H, Maruyama K, Masuhiro Y, Kobayashi Y, Goto M, Tai H, Yanagisawa J, Metzger D, Hashimoto S, Kato S (1999) Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol Cell Biol 19:5363–5372
Rossler OG, Hloch P, Schutz N, Weitzenegger T, Stahl H (2000) Structure and expression of the human p68 RNA helicase gene. Nucleic Acids Res 28:932–939
Nicol SM, Causevic M, Prescott AR, Fuller-Pace FV (2000) The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase. Exp Cell Res 257:272–280
Kitamura A, Nishizuka M, Tominaga K, Tsuchiya T, Nishihara T, Imagawa M (2001) Expression of p68 RNA helicase is closely related to the early stage of adipocyte differentiation of mouse 3T3-L1 cells. Biochem Biophys Res Commun 287:435–439
Liu ZR (2002) p68 RNA helicase is an essential human splicing factor that acts at the U1 snRNA-5’ splice site duplex. Mol Cell Biol 22:5443–5450
Bates GJ, Nicol SM, Wilson BJ, Jacobs AM, Bourdon JC, Wardrop J, Gregory DJ, Lane DP, Perkins ND, Fuller-Pace FV (2005) The DEAD box protein p68: a novel transcriptional coactivator of the p53 tumour suppressor. EMBO J 24:543–553
Camats M, Guil S, Kokolo M, Bach-Elias M (2008) P68 RNA helicase (DDX5) alters activity of cis- and trans-acting factors of the alternative splicing of H-Ras. PLoS ONE 3:e2926
Yang L, Lin C, Liu ZR (2005) Phosphorylations of DEAD box p68 RNA helicase are associated with cancer development and cell proliferation. Mol Cancer Res 3:355–363
Causevic M, Hislop RG, Kernohan NM, Carey FA, Kay RA, Steele RJ, Fuller-Pace FV (2001) Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene 20:7734–7743
Denkberg G, Reiter Y (2006) Recombinant antibodies with T-cell receptor-like specificity: novel tools to study MHC class I presentation. Autoimmun Rev 5:252–257
Cohen CJ, Sarig O, Yamano Y, Tomaru U, Jacobson S, Reiter Y (2003) Direct phenotypic analysis of human MHC class I antigen presentation: visualization, quantitation, and in situ detection of human viral epitopes using peptide-specific, MHC-restricted human recombinant antibodies. J Immunol 170:4349–4361
Acknowledgments
We thank Dr. William P. Weidanz for critical discussion of the data. We thank Dr. Stephen Wright and Dr. Piotr Tabaczewski for his assistance with the mouse models and Dr. Kathryn Norton for tissue acquisition.
Conflict of interest statement
Jon A. Weidanz is Chief Scientist and founder of Receptor Logic, Inc., Abilene, Texas, 79601. Study was supported in part by Receptor Logic, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, B., Hawkins, O.E., Neethling, F.A. et al. Direct discovery and validation of a peptide/MHC epitope expressed in primary human breast cancer cells using a TCRm monoclonal antibody with profound antitumor properties. Cancer Immunol Immunother 59, 563–573 (2010). https://doi.org/10.1007/s00262-009-0774-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0774-8