Abstract
Purpose
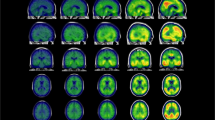
The A/T/N model is a research framework proposed to investigate Alzheimer’s disease (AD) pathological bases (i.e., amyloidosis A, neurofibrillary tangles T, and neurodegeneration N). The application of this system on clinical populations is still limited. The aim of the study is to evaluate the topography of T distribution by 18F-flortaucipir PET in relation to A and N and to describe the A/T/N status through imaging biomarkers in memory clinic patients.
Methods
Eighty-one patients with subjective and objective cognitive impairment were classified as A+/A− and N+/N− through amyloid PET and structural MRI. Tau deposition was compared across A/N subgroups at voxel level. T status was defined through a global cut point based on A/N subgroups and subjects were categorized following the A/T/N model.
Results
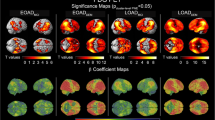
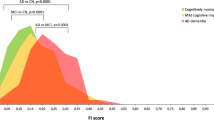
A+N+ and A+N− subgroups showed higher tau burden compared to A−N− group, with A+N− showing significant deposition limited to the medial and lateral temporal regions. Global cut point discriminated A+N+ and A+N− from A−N− subjects. On A/T/N classification, 23% of patients showed a negative biomarker profile, 58% fell within the Alzheimer’s continuum, and 19% of the sample was characterized by non-AD pathologic change.
Conclusion
Medial and lateral temporal regions represent a site of significant tau accumulation in A+ subjects and possibly a useful marker of early clinical changes. This is the first study in which the A/T/N model is applied using 18F-flortaucipir PET in a memory clinic population. The majority of patients showed a profile consistent with the Alzheimer’s continuum, while a minor percentage showed a profile suggestive of possible other neurodegenerative diseases. These results support the applicability of the A/T/N model in clinical practice.


Similar content being viewed by others
References
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47. https://doi.org/10.1212/WNL.0000000000002923.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62. https://doi.org/10.1016/j.jalz.2011.03.004.
Jack CR Jr, Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, et al. Suspected non-Alzheimer disease pathophysiology--concept and controversy. Nat Rev Neurol. 2016;12:117–24. https://doi.org/10.1038/nrneurol.2015.251.
Soldan A, Pettigrew C, Fagan AM, Schindler SE, Moghekar A, Fowler C, et al. ATN profiles among cognitively normal individuals and longitudinal cognitive outcomes. Neurology. 2019;92:e1567–e79. https://doi.org/10.1212/WNL.0000000000007248.
Altomare D, de Wilde A, Ossenkoppele R, Pelkmans W, Bouwman F, Groot C, et al. Applying the ATN scheme in a memory clinic population: The ABIDE project. Neurology. 2019;93:e1635-e46. doi: https://doi.org/10.1212/WNL.0000000000008361
Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, et al. Age-specific and sex-specific prevalence of cerebral beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: a cross-sectional study. Lancet Neurol. 2017;16:435–44. https://doi.org/10.1016/S1474-4422(17)30077-7.
Ekman U, Ferreira D, Westman E. The A/T/N biomarker scheme and patterns of brain atrophy assessed in mild cognitive impairment. Sci Rep. 2018;8:8431. https://doi.org/10.1038/s41598-018-26151-8.
Jack CR Jr, Wiste HJ, Therneau TM, Weigand SD, Knopman DS, Mielke MM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. Jama. 2019;321:2316–25. https://doi.org/10.1001/jama.2019.7437.
Noirot C, Mainta I, Mendes A, Andryszak P, Visvaratnam H, Unschuld PG, et al. Tau PET imaging evidence in patients with cognitive impairment: preparing for clinical use. Clinical and Translational Imaging. 2018;6:471–82. https://doi.org/10.1007/s40336-018-0297-4.
Ossenkoppele R, Rabinovici GD, Smith R, Cho H, Scholl M, Strandberg O, et al. Discriminative accuracy of [18F] flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. Jama. 2018;320:1151–62. https://doi.org/10.1001/jama.2018.12917.
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–52. https://doi.org/10.1016/j.jalz.2014.01.001.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Frisoni GB, Prestia A, Zanetti O, Galluzzi S, Romano M, Cotelli M, et al. Markers of Alzheimer’s disease in a population attending a memory clinic. Alzheimers Dement. 2009;5:307–17. https://doi.org/10.1016/j.jalz.2009.04.1235.
Jovicich J, Marizzoni M, Sala-Llonch R, Bosch B, Bartres-Faz D, Arnold J, et al. Brain morphometry reproducibility in multi-center 3T MRI studies: a comparison of cross-sectional and longitudinal segmentations. Neuroimage. 2013;83:472–84. https://doi.org/10.1016/j.neuroimage.2013.05.007.
Schmidt P. Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging: lmu; 2017.
Shcherbinin S, Schwarz AJ, Joshi A, Navitsky M, Flitter M, Shankle WR, et al. Kinetics of the tau PET tracer 18F-AV-1451 (T807) in subjects with normal cognitive function, mild cognitive impairment, and Alzheimer disease. J Nucl Med. 2016;57:1535–42. https://doi.org/10.2967/jnumed.115.170027.
Schwarz AJ, Shcherbinin S, Slieker LJ, Risacher SL, Charil A, Irizarry MC, et al. Topographic staging of tau positron emission tomography images. Alzheimers Dement (Amst). 2018;10:221–31. https://doi.org/10.1016/j.dadm.2018.01.006.
Mishra S, Gordon BA, Su Y, Christensen J, Friedrichsen K, Jackson K, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage. 2017;161:171–8. https://doi.org/10.1016/j.neuroimage.2017.07.050.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. https://doi.org/10.1006/nimg.2001.0978.
Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72.
Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, et al. Validation of a fully automated 3D hippocampal segmentation method using subjects with Alzheimer’s disease mild cognitive impairment, and elderly controls. Neuroimage. 2008;43:59–68. https://doi.org/10.1016/j.neuroimage.2008.07.003.
Frisoni GB, Redolfi A, Manset D, Rousseau ME, Toga A, Evans AC. Virtual imaging laboratories for marker discovery in neurodegenerative diseases. Nat Rev Neurol. 2011;7:429–38. https://doi.org/10.1038/nrneurol.2011.99.
Redolfi A, McClatchey R, Anjum A, Zijdenbos A, Manset D, Barkhof F, et al. Grid infrastructures for computational neuroscience: the neuGRID example. Future Neurol. 2009;4:703–22.
Bosco P, Redolfi A, Bocchetta M, Ferrari C, Mega A, Galluzzi S, et al. The impact of automated hippocampal volumetry on diagnostic confidence in patients with suspected Alzheimer’s disease: a European Alzheimer’s disease consortium study. Alzheimers Dement. 2017;13:1013–23. https://doi.org/10.1016/j.jalz.2017.01.019.
Pereira JB, Cavallin L, Spulber G, Aguilar C, Mecocci P, Vellas B, et al. Influence of age, disease onset and ApoE4 on visual medial temporal lobe atrophy cut-offs. J Intern Med. 2014;275:317–30. https://doi.org/10.1111/joim.12148.
Rhodius-Meester HFM, Benedictus MR, Wattjes MP, Barkhof F, Scheltens P, Muller M, et al. MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front Aging Neurosci. 2017;9:117. https://doi.org/10.3389/fnagi.2017.00117.
Cotta Ramusino M, Altomare D, Bacchin R, Ingala S, Bna C, Bonetti M, et al. Medial temporal lobe atrophy and posterior atrophy scales normative values. Neuroimage Clin. 2019;24:101936. doi: https://doi.org/10.1016/j.nicl.2019.101936
Pontecorvo MJ, Devous MD Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–63. https://doi.org/10.1093/brain/aww334.
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. https://doi.org/10.1016/S1474-4422(12)70291-0.
Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–48.
Tosun D, Landau S, Aisen PS, Petersen RC, Mintun M, Jagust W, et al. Association between tau deposition and antecedent amyloid-beta accumulation rates in normal and early symptomatic individuals. Brain. 2017;140:1499–512. https://doi.org/10.1093/brain/awx046.
Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S, Swedish Bio F, Jogi J, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain. 2017;140:2286–94. https://doi.org/10.1093/brain/awx171.
Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448–63. https://doi.org/10.1016/j.neuroimage.2017.05.058.
Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:338ra66. https://doi.org/10.1126/scitranslmed.aaf2362.
Bischof GN, Jessen F, Fliessbach K, Dronse J, Hammes J, Neumaier B, et al. Impact of tau and amyloid burden on glucose metabolism in Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3:934–9. https://doi.org/10.1002/acn3.339.
Menkes-Caspi N, Yamin HG, Kellner V, Spires-Jones TL, Cohen D, Stern EA. Pathological tau disrupts ongoing network activity. Neuron. 2015;85:959–66. https://doi.org/10.1016/j.neuron.2015.01.025.
Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain. 2017;140:3286–300. https://doi.org/10.1093/brain/awx243.
Saint-Aubert L, Almkvist O, Chiotis K, Almeida R, Wall A, Nordberg A. Regional tau deposition measured by [(18)F]THK5317 positron emission tomography is associated to cognition via glucose metabolism in Alzheimer’s disease. Alzheimers Res Ther. 2016;8:38. https://doi.org/10.1186/s13195-016-0204-z.
Roberts RO, Knopman DS, Syrjanen JA, Aakre JA, Vassilaki M, Kremers WK, et al. Weighting and standardization of frequencies to determine prevalence of AD imaging biomarkers. Neurology. 2017;89:2039–48. https://doi.org/10.1212/WNL.0000000000004652.
Ferreira D, Cavallin L, Larsson EM, Muehlboeck JS, Mecocci P, Vellas B, et al. Practical cut-offs for visual rating scales of medial temporal, frontal and posterior atrophy in Alzheimer’s disease and mild cognitive impairment. J Intern Med. 2015;278:277–90. https://doi.org/10.1111/joim.12358.
Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87:375–83. https://doi.org/10.1212/WNL.0000000000002892.
Khachaturian AS, Hayden KM, Mielke MM, Tang Y, Lutz MW, Gustafson DR, et al. Future prospects and challenges for Alzheimer’s disease drug development in the era of the NIA-AA research framework. Alzheimers Dement. 2018;14:532–4. https://doi.org/10.1016/j.jalz.2018.03.003.
Jack CR Jr, Therneau TM, Weigand SD, Wiste HJ, Knopman DS, Vemuri P, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer’s Association research framework. JAMA Neurol. 2019. https://doi.org/10.1001/jamaneurol.2019.1971.
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–66. https://doi.org/10.1007/s00401-014-1349-0.
Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–82. https://doi.org/10.1016/j.neuron.2016.01.028.
Mormino EC, Papp KV, Rentz DM, Schultz AP, LaPoint M, Amariglio R, et al. Heterogeneity in suspected non-Alzheimer disease pathophysiology among clinically normal older individuals. JAMA Neurol. 2016;73:1185–91. https://doi.org/10.1001/jamaneurol.2016.2237.
Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59. https://doi.org/10.1146/annurev.neuro.24.1.1121.
Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019. https://doi.org/10.1093/brain/awz099.
Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. https://doi.org/10.1038/35081564.
Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. JAMA Neurol. 2016;73:1070–7. https://doi.org/10.1001/jamaneurol.2016.2078.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Acknowledgments
We thank all patients and volunteers for participating in this study. We thank Avid Radiopharmaceuticals (Lilly) for supplying the precursor for the manufacturing of 18F-flortaucipir.
Funding
The study has been supported by the Swiss National Science Foundation (“The Biological Basis of Cognitive Impairment due to SNAP: Studying the interplay between amyloidosis and tau-related neurodegeneration”—SNF 320030_169876) and partially supported by the Personalized Health and Related Technology Initiative (“Advanced Translational Imaging”—PHRT 2017-512).
This study is part of CoSTREAM (www.costream.eu) and received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 667375.
The Centre de la mémoire at Geneva University Hospital is funded by private donors: A.P.R.A.—Association Suisse pour la Recherche sur la Maladie d’Alzheimer, Genève; Fondation Segré, Genève; Ivan Pictet, Genève; Fondazione Agusta, Lugano; Fondation Chmielewski, Genève.
Author information
Authors and Affiliations
Contributions
Study concept and design: Dodich, Frisoni, Garibotto. Clinical consultant and data acquisition: Mendes, Assal, Chicherio, Rakotomiaramanana, Andryszak, Sheffler, Zekry, Lovblad. Statistical analysis and data interpretation: Dodich, Festari, Ribaldi, Frisoni, Garibotto. Drafting of the manuscript: Dodich, Garibotto. All the authors contributed to the critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee (Commission cantonale d’éthique de la recherche—CCER)) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects participating in this study have signed an informed consent form.
Data policy
The datasets generated during and/or analyzed during the current study are part of the Geneva Memory Clinic dataset and available from the corresponding author on founded request.
Consent for publication
The manuscript has been seen and approved by all authors for submission to EJNMMI.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Dodich, A., Mendes, A., Assal, F. et al. The A/T/N model applied through imaging biomarkers in a memory clinic. Eur J Nucl Med Mol Imaging 47, 247–255 (2020). https://doi.org/10.1007/s00259-019-04536-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04536-9