Abstract
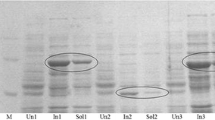
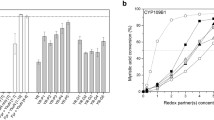
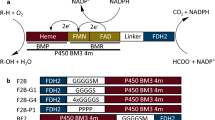
The major limitation in the synthetic application of two-component Baeyer–Villiger monooxygenases was addressed by identifying the 28-kDa flavin-reductase Fre from Escherichia coli as a suitable supplier of reduced FMN for these enzymes. Coexpression of Fre with either 2,5- or 3,6-diketocamphane monooxygenase from Pseudomonas putida NCIMB 10007 significantly enhanced the conversion of camphor and norcamphor serving as representative ketones. With purified enzymes, full conversion was achieved, while only slight amounts of product were formed in the absence of this flavin reductase. Fusion of the genes of Fre and DKCMOs into single open reading frame constructs resulted in unstable proteins exhibiting flavin reducing, but poor oxygenating activity, which led to overall decreased conversion of camphor.



Similar content being viewed by others
References
Balke K, Kadow M, Mallin H, Saß S, Bornscheuer UT (2012) Discovery, application and protein engineering of Baeyer–Villiger monooxygenases for organic synthesis. Org Biomol Chem 10(31):6249–6265
Beecher JE (1997) Sulfoxidation by microbial monooxygenases. Phd Thesis, University of Exeter
Bommarius AS, Drauz K (1994) An enzymatic route to L-ornithine from arginine—activation, selectivity and stabilization of L-arginase. Bioorgan Med Chem 2(7):617–626. doi:10.1016/0968-0896(94)85009-7
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485(7397):185–194. doi:10.1038/nature11117
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72(1–2):248–254. doi:10.1006/abio.1976.9999
Campbell ZT, Baldwin TO (2009) Fre is the major flavin reductase supporting bioluminescence from Vibrio harveyi luciferase in. J Biol Chem 284(13):8322–8328. doi:10.1074/jbc.M808977200
Chung CT, Niemela SL, Miller, RH (1989) One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 86(7):2172–2175
Conrad HE, DuBus R, Namvedt M, Gunsalus IC (1965a) Mixed function oxidations II: separation and properties of the enzymes catalyzing camphor lactonization. J Biol Chem 240(1):495–503
Conrad HE, Lieb K, Gunsalus IC (1965b) Mixed function oxidation III: an electron transport complex in camphor ketolactonization. J Biol Chem 240(10):4029–4037
de Gonzalo G, Mihovilovic MD, Fraaije MW (2010) Recent developments in the application of Baeyer–Villiger monooxygenases as biocatalysts. ChemBioChem 11(16):2208–2231. doi:10.1002/cbic.201000395
Donoghue NA, Norris DB, Trudgill PW (1976) The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL1 and Acinetobacter NCIMB 9871. Eur J Biochem 63(1):175–192
Ellis HR (2010) The FMN-dependent two-component monooxygenase systems. Arch Biochem Biophys 497(1–2):1–12
Fink MJ, Rial DV, Kapitanova P, Lengar A, Rehdorf J, Cheng Q, Rudroff F, Mihovilovic MD (2012) Quantitative comparison of chiral catalysts selectivity and performance: a generic concept illustrated with cyclododecanone monooxygenase as Baeyer–Villiger biocatalyst. Adv Synth Catal 354:3491–3500. doi:10.1002/adsc.201200453
Fontecave M, Eliasson R, Reichard P (1987) NAD(P)H:flavin oxidoreductase of Escherichia coli. A ferric iron reductase participating in the generation of the free radical of ribonucleotide reductase. J Biol Chem 262(25):12325–12331
Gagnon R, Grogan G, Groussain E, Pedragosa-Moreau S, Richardson PF, Roberts SM, Willetts AJ, Alphand V, Lebreton J, Furstoss R (1995) Oxidation of some prochiral 3-substituted cyclobutanones using monooxygenase enzymes: a single-step method for the synthesis of optically enriched 3-substituted γ-lactones. J Chem Soc Perkin Trans I:2527–2528
Gagnon R, Grogan G, Wan P, Levitt MS, Peter MR, Willetts AJ (1994) Biological Baeyer–Villiger oxidation of some monocyclic and bicyclic ketones using monooxygenases from Acinetobacter calcoaceticus NCIMB 9871 and Pseudomonas putida NCIMB 10007. J Chem Soc Perkin Trans I:2537–2543
Gibson QH, Hastings JW (1962) Oxidation of reduced flavin mononucleotide by molecular oxygen. Biochem J 83(2):368–377
Grogan G (1995) Microbial biotransformations: Oxygenation of cyclic ketones by Baeyer–Villiger monooxygenases from camphor-grown Pseudomonas putida NCIMB 10007. PhD thesis, University of Exeter
Grogan G, Roberts SM, Wan P, Willetts AJ (1993) Some Baeyer–Villiger oxidations using a monooxygenase enzyme from Pseudomonas putida NCIMB 10007. J Chem Soc Chem Commun 10(8):699–701. doi:10.1039/c39930000699
Gunsalus IC, Conrad HE, Trudgill PW, Jacobson LA (1965) Regulation of catabolic metabolism. Israel J Med Sci 1(6):1099–1119
Hanes CS (1932) Studies on plant amylases: the effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J 26:1406–1421
Harayama S, Kok M, Neidle EL (1992) Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol 46(1):565–601. doi:10.1146/annurev.mi.46.100192.003025
Hastings JW, Potrikus CJ, Gupta SC, Kurfurst M, Makemson JC (1985) Biochemistry and physiology of bioluminescent bacteria. Adv Microb Physiol 26:235–291. doi:10.1016/s0065-2911(08)60398-7
Horowitz NH (1945) On the evolution of biochemical syntheses. Proc Natl Acad Sci USA 31(6):153–157. doi:10.1073/pnas.31.6.153
Ingelman M, Ramaswamy S, Nivière V, Fontecave M, Eklund H (1999) Crystal structure of NADPH:flavin oxidoreductase from Escherichia coli. Biochemistry 38(22):7040–7049. doi:10.1021/bi982849m
Iwaki H, Grosse S, Bergeron H, Leisch H, Morley K, Hasegawa Y, Lau PCK (2013) Camphor pathway redux: functional recombinant expression of 2,5- and 3,6-diketocamphane monooxygenases of Pseudomonas putida ATCC 17453 with their cognate flavin reductase catalyzing Baeyer–Villiger reactions. Appl Environ Microbiol 79(10):3282–3293. doi:10.1128/aem.03958-12
Jawanda N, Ahmed K, Tu S-C (2007) Vibrio harveyi flavin reductase−luciferase fusion protein mimics a single-component bifunctional monooxygenase. Biochemistry 47(1):368–377. doi:10.1021/bi701392b
Jones KH, Smith RT, Trudgill PW (1993) Diketocamphane enantiomer-specific Baeyer–Villiger monooxygenases from camphor-grown Pseudomonas putida ATCC 17453. J Gen Microbiol 139:797–805
Kadow M, Loschinski K, Saß S, Schmidt M, Bornscheuer U (2012) Completing the series of BVMOs involved in camphor metabolism of Pseudomonas putida NCIMB 10007 by identification of the two missing genes, their functional expression in E. coli, and biochemical characterization. Appl Microbiol Biotechnol 96(2):419–429. doi:10.1007/s00253-011-3859-1
Kadow M, Sass S, Schmidt M, Bornscheuer UT (2011) Recombinant expression and purification of the 2,5-diketocamphane 1,2-monooxygenase from the camphor metabolizing Pseudomonas putida strain NCIMB 10007. AMB Express 1(1):13. doi:10.1186/2191-0855-1-13
Klaassen PD, Vollebregt AWHN, Van Den Berg MAP, Hans MDH, Van Der Laan JMB (2010) Process for preparing pravastatin. US Patent US 2010/0217032 A1
Koga H, Yamaguchi E, Matsunaga K, Aramaki H, Horiuchi T (1989) Cloning and nucleotide sequences of NADH-putidaredoxin reductase gene (camA) and putidaredoxin gene (camB) involved in cytochrome P-450cam hydroxylase of Pseudomonas putida. J Biochem 106(5):831–836
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. doi:10.1093/bioinformatics/btm404
Leisch H, Morley K, Lau PCK (2011) Baeyer−Villiger monooxygenases: more than just green chemistry. Chem Rev 111(7):4165–4222. doi:10.1021/cr1003437
Massey V (1994) Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem 269(36):22459–22462
Matsuoka T, Miyakoshi S, Tanzawa K, Nakahara K, Hosobuchi M, Serizawa N (1989) Purification and characterization of cytochrome P-450sca from Streptomyces carbophilus. Eur J Biochem 184(3):707–713. doi:10.1111/j.1432-1033.1989.tb15070.x
McGhie EJ (1998) Studies on monooxygenases from the camphor degradation pathway in Pseudomonas putida NCIMB 10007. PhD thesis, University of Exeter
McGhie EJ, Isupov MN, Schröder E, Littlechild JA (1998) Crystallization and preliminary X-ray diffraction studies of the oxygenating subunit of 3,6-diketocamphane monooxygenase from Pseudomonas putida. Acta Crystallogr Sect D: Biol Crystallogr D54:1036–1038
Meighen EA (1991) Molecular biology of bacterial bioluminescence. Microbiol Rev 55(1):123–142
Mirza IA, Yachnin BJ, Wang S, Grosse S, Hln B, Imura A, Iwaki H, Hasegawa Y, Lau PCK, Berghuis AM (2009) Crystal structures of cyclohexanone monooxygenase reveal complex domain movements and a sliding cofactor. J Am Chem Soc 131(25):8848–8854. doi:10.1021/ja9010578
Nijvipakul S, Wongratana J, Suadee C, Entsch B, Ballou DP, Chaiyen P (2008) LuxG is a functioning flavin reductase for bacterial luminescence. J Bacteriol 190(5):1531–1538. doi:10.1128/jb.01660-07
Nivière V, Fieschi F, Décout J-L, Fontecave M (1996) Is the NADPH:flavin oxidoreductase from Escherichia coli a member of the ferredoxin-NADP+ reductase family? J Biol Chem 271(28):16656–16661. doi:10.1074/jbc.271.28.16656
Nodate M, Kubota M, Misawa N (2006) Functional expression system for cytochrome P450 genes using the reductase domain of self-sufficient P450RhF from Rhodococcus sp NCIMB 9784. Appl Microbiol Biotechnol 71(4):455–462. doi:10.1007/s00253-005-0147-y
Palchaudhuri S, Chakrabarty A (1976) Isolation of plasmid deoxyribonucleic-acid from Pseudomonas putida. J Bacteriol 126(1):410–416
Payne JW, Bolton H, Campbell JA, Xun LY (1998) Purification and characterization of EDTA monooxygenase from the EDTA-degrading bacterium BNC1. J Bacteriol 180(15):3823–3827
Rheinwald JG, Chakrabarty AM, Gunsalus IC (1973) A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A 70(3):885–9
Robinson CR, Sauer RT (1998) Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc Natl Acad Sci U S A 95(11):5929–5934. doi:10.1073/pnas.95.11.5929
Serizawa N, Matsuoka T (1991) A 2 component-type cytochrome-P-450 monooxygenase system in a prokaryote that catalyzes hydroxylation of ML-236B to pravastatin, a tissue-selective inhibotor of 3-hydroxy-3-methylglutaryl coenzyme-A reductase. Biochim Biophys Acta 1084(1):35–40. doi:10.1016/0005-2760(91)90052-j
Spyrou G, Haggård-Ljungquist E, Krook M, Jörnvall H, Nilsson E, Reichard P (1991) Characterization of the flavin reductase gene (fre) of Escherichia coli and construction of a plasmid for overproduction of the enzyme. J Bacteriol 173(12):3673–3679
Taylor DG, Trudgill PW (1986) Camphor revisited: Studies of 2,5-diketocamphane 1,2-monooxygenase from Pseudomonas putida ATCC 17453. J Bacteriol 165(8):489–497
Torres Pazmiño DE, Snajdrova R, Baas B-J, Ghobrial M, Mihovilovic MD, Fraaije MW (2008) Self-sufficient Baeyer–Villiger monooxygenases: effective coenzyme regeneration for biooxygenation by fusion engineering. Angew Chem Int Ed 47:2275–2278. doi:10.1002/anie.200704630
Torres Pazmiño DE, Riebel A, de Lange J, Rudroff F, Mihovilovic MD, Fraaije MW (2009) Efficient biooxidations catalyzed by a new generation of self-sufficient Baeyer–Villiger monooxygenases. ChemBioChem 10:2595–2598. doi:10.1002/cbic.200900480
Trudgill PW, DuBus R, Gunsalus IC (1966) Mixed function oxidation VI. Purification of a tightly coupled electron transport complex in camphor lactonization. J Biol Chem 241(18):4288–90
van Berkel WJ, Kamerbeek NM, Fraaije MW (2006) Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J Biotechnol 124(4):670–689
Villa R, Willetts A (1997) Oxidations by microbial NADH plus FMN-dependent luciferases from Photobacterium phosphoreum and Vibrio fischeri. J Mol Catal B Enzym 2:193–197
Walsh CT, Wencewicz TA (2013) Flavoenzymes: versatile catalysts in biosynthetic pathways. Nat Prod Rep 30(1):175–200. doi:10.1039/c2np20069d
Willetts A (1997) Structural studies and synthetic applications of Baeyer–Villiger monooxygenases. Trends Biotechnol 15(2):55–62. doi:10.1016/s0167-7799(97)84204-7
Williams DR, Trudgill PW, Taylor DG (1989) Metabolism of 1,8-cineole by a Rhodococcus species: ring cleavage reactions. J Gen Microbiol 135(7):1957–1967. doi:10.1099/00221287-135-7-1957
Zenno S, Saigo K (1994) Identification of the genes encoding NAD(P)H-flavin oxidoreductases that are similar in sequence to Escherichia coli Fre in four species of luminous bacteria: Photorhabdus luminescens, Vibrio fischeri, Vibrio harveyi, and Vibrio orientalis. J Bacteriol 176(12):3544–3551
Acknowledgments
We thank the European Research Council (ERC AdG 247014), the Swedish Research Council, the Deutsche Forschungsgemeinschaft (Grant Bo1862/6-1) and the Deutsche Bundesstiftung Umwelt (AZ13234 and AZ20013/231) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadow, M., Balke, K., Willetts, A. et al. Functional assembly of camphor converting two-component Baeyer–Villiger monooxygenases with a flavin reductase from E. coli . Appl Microbiol Biotechnol 98, 3975–3986 (2014). https://doi.org/10.1007/s00253-013-5338-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5338-3