Abstract
Our objective was to improve the results of extracorporeal shock waves lithotripsy using hydroxycitric acid (HCA) like adjuvant therapy. Double blind randomized clinical trial using hydroxycitric acid versus placebo (ID NCT05525130). Multicenter study of adjuvant exposure to a food supplement with hydroxycitric acid (HCA), vs. placebo in patients with calcium oxalate and calcium phosphate lithiasis with indication for extracorporeal shock wave lithotripsy (ESWL). 81 patients were included in the study to compare the effect of HCA versus placebo. Stone fragmentation, the main efficacy variable. Other variables analyzed were stone size, Hounsfield Unit Stone and tolerability. Statistical study with SPSS, statistical significance p ≤ 0.05. Eighty-one patients were included, 40 in the intervention group with HCA and 41 in the control group with placebo. The average stone area was 174,36 mm2 (SD: 32,83 mm2) and the average hardness was 1128,11 (SD: 257,65), with no statistically significant differences between groups. Significant statistical differences were obtained in the analysis of the population by intention to treat and by protocol of the main variable, no fragmentation vs. fragmentation where 100% of the patients, who were given ESWL and took HCA, presented fragmentation while 17% of the patients with placebo did not reach fragmentation (p = 0.03). The adjuvant use of HCA in patients for whom ESWL has been indicated, facilitates stone fragmentation in all cases, which is not achieved in up to 17% of the patients who did not use HCA. We recommend the use of HCA in patients prior to shock wave treatment to improve their fragmentation in calcium stones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrolithiasis is characterized by the presence of stones in the upper urinary tract. It is a common pathology in our society, affecting 1–15% of the population [1], which has experienced an increase in its incidence in recent decades. Moreover, nephrolithiasis is a pathology with a high recurrence rate: it is estimated that the risk of recurrence of untreated nephrolithiasis is approximately 15% in the first year, 35% at 5 years and more than 50% at 10 years [2]. It is estimated that the main chemical compound involved in 60% of the lithiasis cases is calcium oxalate, whereas calcium phosphate (hydroxyapatite) is involved in 20% of the cases, uric acid in 7% of the cases, struvite lithiasis (ammonium-magnesium phosphate) in 7% of the cases and the remaining 6% involve other less frequent compounds such as cystine [1].
Therefore, calcium oxalate and calcium phosphate stones are the most prevalent. These occur when, due to metabolic alterations and/or exogenous factors, the urine becomes saturated with elements that promote the aggregation and the precipitation of these crystals. Hypercalciuria is the most frequent identified metabolic alteration in patients with this type of renal stones and it is estimated that 35–65% of patients with nephrolithiasis present this type of alteration [3]. Substances that inhibit the stone formation, such as citrate, can also be found in urine and the reduction of citrate (hypocitraturia) is also a risk factor for the stone formation. Other factors involved in the formation of calcium oxalate and calcium phosphate stones/s are a low urinary volume, hypomagnesuria and hyperuricosuria [4].
Extracorporeal shock wave lithotripsy (ESWL) is the least invasive treatment for most nephrolithiasis regardless of where the stone is located. When properly indicated, this technique allows very satisfactory results to be achieved. The correct selection of the procedure for stone removal will depend on the characteristics of the stone (size, radiological density and location) as well as other factors (body mass index (BMI), skin-renal stone distance, skeletal deformities, bleeding diathesis, pregnancy, urinary tract anatomy). The stone size is the most important factor influencing the outcome of ESWL. The European Association of Urology recommends ESWL as the first treatment option in cases of renal stones smaller than 1 cm [5].
Although ESWL is a technique with many advantages over other techniques, e.g. less invasive, intravenous sedation with good tolerance and favourable safety profile, the use of other complementary procedures can improve the success rate of ESWL in less favourable cases of renal stones. Therefore, research on these adjunctive treatments to ESWL is needed. FagolitosPlus® is a food supplement whose main ingredient is hydroxycitric acid (HCA). HCA has shown an in vitro inhibitory effect on the calcium oxalate monohydrate (OCM) crystals growth, even in highly supersaturated solutions [6]. In addition, both citrate (CA) and HCA show an inhibitory effect on the calcium phosphate crystals growth. HCA results in electrostatic interactions with calcium ions, forming hydrogen bonds with calcium oxalate and calcium phosphate crystals and then inhibiting the nucleation and growth of these crystals.
In addition, the product contains pyridoxine (vitamin B6), which has a reducing effect on the production of endogenous oxalate by increasing glyoxalate transamination (oxalate precursor) to glycine [7]. It also contains magnesium, which is not only capable of binding to oxalate in urine preventing from binding with calcium but which can also reduce the intestinal absorption of calcium [8].
Based on the above as well as on a preliminary retrospective study published on the results of HCA [9] and the in vitro results of HCA on oxalate and calcium phosphate, we believe that an in vivo experimental study is needed to test the effects of HCA on the efficacy and safety of ESWL.
Material and methods
Type of study
Experimental, longitudinal, prospective, two-arm, randomized, double-blind, multicenter design study of an adjuvant exposure to a food supplement, HCA vs placebo in patients with oxalate and calcium phosphate stones to whom extracorporeal shock wave lithotripsy (ESWL) was indicated. (Trial number protocol is ARA-EXP/FAG-2019–03). This study has been registered in ClinicalTrials.gov (ID NCT05525130). Randomization to the study treatment groups was performed by using a randomization software programme based on an initial seed that generated a random distribution of blocks and individuals into two groups in a 1:1 ratio.
Methodology and plan of the experimental study
The administration period of HCA/Placebo was 2.5 months, starting 4 weeks prior to ESWL until 6 weeks after ESWL. The HCA/placebo regimen was of one sachet every 12 h for 4 weeks uninterrupted until ESWL, and then one sachet of HCA/placebo per day uninterrupted for 6 weeks after ESWL. No instrumental or surgery treatment was performed within 6 weeks after the primary SWL treatment and that results of retreatments after 6 weeks were not included in the analysis.
Inclusion criteria
The study population included patients of both sexes and aged 18 years old and over with raquiopaque renal stones with a size of 175 mm2 ± 25 mm2 (diameters between 1.5 × 1 cm and 2 × 1 cm) and more than 850 Hounsfield Unit and negative urine culture. Participants were also symptomatic with a low probability of spontaneous expulsion (Usually stone diameter > 6 mm has low probability of spontaneous expulsion). After the investigator informed about the study, the patients who agreed to participate in the clinical study signed the informed consent form.
Exclusion criteria
Patients with nephrolithiasis larger than 200 mm2 and smaller than 150 mm2 were excluded, as well as patients taking pharmaceutical treatments producing or inhibiting the formation of urinary stones (citrate, bicarbonate, thiazides, bisphosphonates, magnesium, calcium, vitamin B6, vitamin D, vitamin A, xanthine oxidase inhibitors, uricosurics, probiotics, urease inhibitors, cystine chelators, topiramate, antiretrovirals), or patients taking medicines influencing urine pH (citrate, bicarbonate, carbonic anhydrase inhibitors, l-methionine, vitamin C, ammonium chloride) or antidepressants of the selective serotonin reuptake inhibitors group (risk of interaction with the product) or anticoagulants or antiplatelet medicines [10]. Patients with active or recurrent urinary tract infections or bleeding diathesis were also excluded. Finally, pregnant or breastfeeding women, as well as other contraindications to the use of ESWL were also excluded from the study (severe obesity, arterial aneurysm close to the location of the stone, etc.).
Study product
The product is a food supplement that contains a granulate in sachets, consisting of 2069.92 mg (0.0021 kg) of HCA (Garcinia Cambogia extract), 200 mg (0.0002 kg) of magnesium, 25 mg (0.000025 kg) of vitamin B6, 10 mg (0.000010 kg) of zinc and 800 µg (0.00000008 kg) vitamin A in each sachet orally administered. It consists of a sustained-release formulation that aims to achieve adequate levels of the components in urine for 24 h. It is manufactured by ARAFARMA GROUP, S.A. and is authorized for marketing in Spain.
Variables analysis
Gender (male/female), age (years), body mass index (kilograms/ m2) were recorded at the initial visit. In order to confirm the presence of kidney stones and their possible modifications (number, location, size or renal stone burden, reduction/growth, etc.), CT imaging studies were performed at the initial and final visits, and a simple radiology of the urinary tract was performed at the third visit, in accordance with the usual clinical practice of the investigational sites. To assess treatment compliance, the percentage of sachets used was calculated and the causes of non-compliance were recorded. All morbidities associated with ESWL understood as clinical problems or complications occurring 2 and 6 weeks after ESWL were recorded. Stone fragmentation, which is the primary efficacy variable, was categorized as follows: no fragmentation (one stone, no volume difference), good fragmentation (≥ 2 stone fragments, and < 50% expulsion or less than 50% volume reduction), very good fragmentation (multiple stone fragments, ≥ 2 fragments with > 50% expulsion or more than 50% volume reduction) and complete fragmentation (absence of renal stones or non-significant residual fragments, < 4 mm). Sample size was calculated for two independent proportions (% of fragmentation). Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, 40 subjects were necessary in the first group and 40 in the second one to obtain statistically significant differences, expected to be of 0 in group 1 and 0.2 in group 2. A drop-out rate of 1% had been anticipated. (The ARCSINUS approximation). Data were obtained from a previous study [1].
Four study visits were completed by each patient. The second visit took place 4 weeks after the beginning of the study, when the ESWL was performed, and the third and fourth visits were completed 2 and 6 weeks after the ESWL was performed, respectively. Only one session of ESWL was performed for each patient.
Protocol of ESWL
The protocol of ESWL is similar in the three centers. Medication with 50 mg of petidine plus 8 mg of ondansetron and 50 mg of dexketoprofen. Blood pressure and cardiac frecuency controlled in all moment. Number of shock waves between 2500 and 3500 with maximum energy of 160 kV.
Objectives
The primary objective of the study was to assess the effects of adjuvant HCA versus adjuvant placebo on the efficacy (fragmentation) of ESWL in calcium oxalate and calcium phosphate urinary stones, when administered for 4 weeks before ESWL and 6 weeks after ESWL. The scientific aim of the study was to demonstrate if modifications of crystal structure by treatment may imply a reduced resistance to SWL Secondary objectives were to evaluate investigational treatment compliance and to assess the safety (morbidities) of ESWL at 2 and 6 weeks after ESWL.
Statistical study
Statistical analysis was performed using the IBM SPSS v. 25.0 Statistical package. To assess the main study variable, the per-protocol population (PP) was used, defined as the population that did not discontinue the study and achieved more than 80% adherence to the food supplement/placebo. The intention-to-treat (ITT) population was defined as all the patients who participated in the study and who, once randomized, took at least one dose of the food supplement or placebo. The chi-square test and Fisher's exact test were used for the comparison of qualitative and quantitative variables and the t-student test was used for the analysis of quantitative variables. Statistical significance was considered p ≤ 0.05.
Ethical considerations
The Spanish Agency of Medicines and Health Products decided to classify the study as a “non-observational study without investigational drugs”. The clinical study was also approved by the Ethics Committee of the San Cecilio Clinical University Hospital of Granada (Spain) in December 2019. Along with this investigational site, two other sites also participated: The Regional University Hospital of Málaga (Spain) and The Virgen de Valme University Hospital of Sevilla (Spain).
Results
A total of 81 patients (ITT) entered the study: 40 were assigned to the HCA interventional group and 41 to the placebo control group (Table 1). 49% were male (mean age 52.07, SD: 11.57 years) and 51% were female (mean age 51.49, SD: 11.14 years). The mean BMI of the study patients was of 28.55 kg/m2 (Tables 2 and 3).
There were no statistical differences in the distribution of gender of the ITT population (Chi-square, Fisher’s exact test: 0.66) or age (F = 0.93, p = 0.34), between both treatment arms of the study. The average stone area was 174.36 mm2 (SD: 32.83 mm2) and the average hardness was 1128.11(SD: 257.65) with no statistically significant differences between both treatment groups (area, F 0.84 p = 0.36; hardness, F 0.08 p = 0.77). 59% of the stones were located in the right kidney. According to the location in the kidney, 27.5% of the stones were located in the lower calyx, 62.5% in the renal pelvis, 8.75% in the upper calyx and 1.25% in the ureteropelvic junction. No differences were observed in eGFR between groups (control group 97.67 ± 5.25 ml/min; experimental group 96.55 ± 6.43 ml/min).
The treatment compliance of the ITT population was of 92%, 95% in the control group and 88% in the experimental group, and no statistically significant differences were found (p < 0.05). Of the 81 patients who entered the study, 6 discontinued before the end of the study. The reasons for discontinuation are shown in Table 4. 65 patients had treatment compliance of more than 80%, being this the population defined as per protocol (PP). Among the patients with < 80% of treatment compliance, the main reason was digestive discomfort in 4 of the patients, and other reasons were forgetfulness or COVID-19.
Of the 65 patients in the PP analysis population (Tables 1 and 2), with a mean age of 53.18 years (SD: 12.27), 508.% were male (mean age 53.61, SD: 11.76 years) and 49.2% were female (mean age 52.75, SD: 10.07 years). The mean BMI of the study patients was 28.06 kg/m2. There were no statistical differences in the gender distribution (Chi-square, Fisher's exact test: 0.80) or age (F = 0.05, p = 0.82) between both study arms. The average stone area was 173.92 mm2 (SD: 33.67 mm2) and the average hardness was 1150.10 (SD: 251.46), with no statistically significant differences between both treatment groups (area, F 0.43 p = 0.84; hardness, F 0.03 p = 0.87). Of the 35 patients whose stone composition could be analyzed, 76.86% of the average stone composition was calcium oxalate [18 stones analyzed in experimental group (11 monohydrate calcium oxalate, 4 calcium phosphate (2 apatite and 2 brushite), 3 dihydrate calcium oxalate). 17 stones analyzed in placebo group (10 monohydrate calcium oxalate, 5 calcium phosphate (3 brushite and 2 apatite), 2 dihydrate calcium oxalate. Method used for Stone composition was infrared spectrometry].
In the PP population, the average number of shock waves used was 2939.58 (SD: 375.08) with an average shock wave frequency of 76.02 Hz (SD: 12.97 Hz) and an energy setting of 135.24 kV (SD: 16.63 kV) with no statistically significant differences between both treatment groups (F 2.45 p = 0.12; F 0.005 p = 0.95; F 0.16 p = 0.69 respectively). In the ITT population, no statistically significant differences were found between groups (mean 2929.02 Ds 412.87 F 1.58 p = 0.21; mean 76.26 Hz Ds 12.76 Hz F 0.04 p = 0.84; mean 135.22 kv Ds 16.78 kV F 0.54 p = 0.82 respectively).
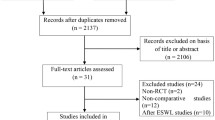
For the main study variable in the PP analysis, the intensity of fragmentation (no fragmentation, good, very good or complete) was distributed differently in both study treatment arms (Chi-square p = 0.028), Table 5. The same results were obtained in the ITT analysis (Chi-square p = 0.011). When we group the intensity of fragmentation into subgroups of two, the only group in which statistically significant differences were obtained in the PP and ITT analysis (Chi-square, Fisher's exact test p = 0.026 and p = 0.006 respectively) was the association of no fragmentation vs. fragmentation (good, very good or complete). All the patients who had undergone ESWL and who took HCA presented fragmentation, while 16.67% (PP) of the patients in the placebo group did not achieve fragmentation of the stones with ESWL (Table 6), 18.42% in the ITT population (Fig. 1).
When assessing the need for a new therapeutic intervention in the PP analysis after ESWL, there was a 1.44% difference in favour of the HCA group (60.71%) versus the placebo group (62.16%). However, this difference was not statistically significant (Chi-square p 0.42). 17 patients in the placebo group and 13 in the HCA group required a new ESWL.
After lithotripsy, few adverse events were observed in the following 2 weeks, with no statistically significant differences between the two exposure groups (Table 7).
Discussion
The food supplement known as FagolitosPlus® is mainly composed of HCA, an ingredient that was identified in 1965 as a component of the Garcinia Cambogia [11] fruit. In vitro assays reveal that HCA is capable of dissolving calcium oxalate monohydrate (COM) and brushite crystals. This phenomenon was observed under specific conditions—namely under continuous flow of a growth solution containing a relatively narrow range of HCA concentration [6, 12,13,14,15]. The product releases a constant amount of HCA over 24 h, through an extended-release mechanism, by generating a constant flow of HCA that reaches the urine. Under these conditions we assimilated the mechanism observed in vitro. This facilitated the fragmentation of COM crystals [6]. This compound had important in vitro properties, especially its ability to bind calcium (in the same way as the citrate does) by inhibiting its crystallization and weakening the molecular union of calcium oxalate and calcium phosphate crystals [12]. In addition to this, different in vitro studies demonstrated that HCA is capable of dissolving calcium oxalate crystals in a supersaturated solution. HCA is excreted in human urine without metabolism and without modifying the urinary pH, compared with citrate, which makes it particularly interesting for its properties in patients with calcium oxalate and phosphate urolithiasis [6, 13, 14]. Experimental animal studies demonstrated the effect of HCA on the inhibition of the crystallization of calcium oxalate, and in addition, its renal elimination. Therefore, HCA has a double effect: a prophylactic effect in the first case and a therapeutic effect in the second one [15, 16].
In the clinical study carried out, the adjuvant use of HCA in patients with an indication to undergo ESWL and who took two sachets daily for one month prior to ESWL, and one sachet daily for 45 days after ESWL, facilitated the stone fragmentation in all cases. The stone fragmentation was not achieved in up to 16.67% of patients who did not take HCA in stones, especially those composed of calcium oxalate (76.86%), and also presented high hardness conditions (mean: 1150.10 DS: 251.46). The improvement of the stone fragmentation in the HCA adjuvant group was obtained both in the 81 patients (intention-to-treat) and in the per protocol population (65 patients). The results of this clinical study confirm the results obtained in a preliminary study, which was conducted by our group and also previously published, in which a higher percentage of stone fragmentation with extracorporeal shock waves was observed in those patients HCA prior to treatment [9].
In addition, a slight decrease of new therapeutic intervention with ESWL (6.93% less) was observed in the group treated with HCA compared with the control group. Therefore, there is a greater fragmentation, and the need for retreatment in patients with calcium lithiasis treated medically with this food supplement based on HCA may decrease. We found that the adherence to HCA of 87.86% of patients is very good. This is the first randomized clinical study with this food supplement compared to placebo which demonstrates the optimization of the fragmentation due to ESWL. These results should be confirmed by studies carried out in other sites in the future. This study has some limitations as absence of 24-h urine testing and only analyze the short-term effect of the drug in ESWL treatment but not in long-term metabolic effects.
Conclusion
The use of the food supplement FagolitosPlus® improves the fragmentation of calcium urolithiasis achieved by using ESWL, so we recommend the use of this product prior to the treatment with ESWL and also after this treatment has ended.
Data availability
Not available.
References
Pearle MS, Antonelli JA, Lotan Y (2016) Urinary lithiasis: etiology, epidemiology and pathogenesis. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds) Campbell-Walsh urology, vol 2. Elsevier, Philadelphia
Uribarri J, Oh MS, Carroll HJ (1989) The first kidney stone. Ann Intern Med 111:1006–1009
Levy FL, Adams-Huet B, Pak CY (1995) Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med 98:50–59
González G (2013) Nephrolithiasis: study and endocrocrinological management. Rev Med Clin 24:798–803
Orihuela-Arroyo B, Arrabal-Polo MA, Arrabal-Martín M (2018) Tratamiento de la Litiasis Renal en Cáliz Inferior. Litotricia Extracorpórea Versus Cirugía Intrarrenal Retrógada o Percutánea. Actual Med 103:66–71
Chung J, Granja I, Taylor MG, Mpourmpakis G, Asplin JR, Rimer JD (2016) Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature 536:446–450
Curhan GC, Willett WC, Speizer FE, Stampfer MJ (1999) Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 25:85–91
Taylor EN, Curhan GC (2006) Diet and fluid prescription in stone disease. Kidney Int 20:1–6
Morales-Martínez A, Teresa Melgarejo-Segura M, Carmen Cano-García del M, Gutiérrez-Tejero F, Arrabal-Martín M, Ángel Arrabal-Polo M (2021) Evaluación del tratamiento de litiasis renal radiopaca mediante la combinación de litotricia extracorpórea por ondas de choque y Fagolitos Plus®. Estudio preliminar de casos y controles. Arch Esp Urol 74:489–493
Tres JC (2006) Interaction between medicines and medicinal plants. An Sist Sanit Navar 29:233–252
Lewis Y, Neelakantan S (1965) Hydroxycitric acid-the principal acid in the fruits of Garcinia cambogia desr. Phytochemistry 4:619–625
- Lancina Martín JA, Salinas Casado J, Rodríguez Miñón JL, González Enguita C (2019). Tratamiento médico de la litiasis urinaria. Urol Integr Invest. 29 p.
Kim D, Rimer JD, Asplin JR (2019) Hydroxycitrate: a potential new therapy for calcium urolithiasis. Urolithiasis 47:311–320
Kyada A, Mansuri N, Patel P (2017) In vitro investigation of some alternative therapeutic agents for antiurolithiatic activity. J Pharm Res 11:955–961
Chen WC, Chen HY, Lin WY, Yang YR, Tsai MY, Chen YH (2018) Inhibitory effect of hydroxycitrate on calcium oxalate crystal formation in a Drosophila model. J Food Nutr Res 6:706–709
Kyada A, Mansuri N, Patel P (2017) Protective effect of magnesium lactate gluconate and Garcinia cambogia fruit extract in experimentally induce renal calculi in rats. J Intercult Ethnopharmacol 6:378–384
Author information
Authors and Affiliations
Contributions
Cano García and Arrabal Polo wrote manuscript Cano Garcia, Caballero Cobos, Vadillo Bohorquez, Molina Diaz follow up patients during clinical trial and are main investigators Yañez Castillo and Gutierrez Tejero prepared figure and tables Arrabal Martin and Reina Ruiz reviewed manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
del Carmen Cano García, M., Cobos, R.C., Bohorquez, Á.V. et al. A randomized, double-blind, placebo-controlled clinical trial of the use of hydroxycitric acid adjuvant to shock wave lithotripsy therapy in patients with calcium stones. Stone fragmentation results. Urolithiasis 51, 83 (2023). https://doi.org/10.1007/s00240-023-01456-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-023-01456-0