Abstract
Diphenylarsinic acid (DPAA) is an organic arsenic compound used for the synthesis of chemical weapons. We previously found that the residents of Kamisu city in Ibaraki Prefecture, Japan, were exposed to DPAA through contaminated well water in 2003. Although mounting evidence strongly suggests that their neurological symptoms were caused by DPAA, the dynamics of DPAA distribution and metabolism after ingestion by humans remain to be elucidated. To accurately predict the distribution of DPAA in the human body, we administrated DPAA (1.0 mg/kg/day) to cynomolgus monkeys (n = 28) for 28 days. The whole tissues from these monkeys were collected at 5, 29, 170, and 339 days after the last administration. The concentration of DPAA in these tissues was measured by liquid chromatography–mass spectrometry. We found that DPAA accumulated in the central nervous system tissues for a longer period than in other tissues. This finding would extend our knowledge on the distribution dynamics and metabolism of DPAA in primates, including humans. Furthermore, it may be useful for developing a treatment strategy for patients who are exposed to DPAA.
Similar content being viewed by others
Introduction
Seaweed contains a large number of organic arsenic compounds, including non-toxic compounds, and many toxic compounds are synthesized as pharmaceutical and chemical warfare agents. Diphenylarsinic acid (DPAA) is an organic arsenic compound that was used for the synthesis of chemical weapons during World War II (Kurata 1980). In 2003, several patients with illnesses of unknown origin were reported in Ibaraki Prefecture, Japan. Organic arsenic compounds that were found in the wells of Kamisu city in Ibaraki were identified as the cause of these symptoms (Ishii et al. 2004). Contaminated water from one of the wells contained 4.5 mg As/L of DPAA, which is approximately 450 times higher than the concentration permitted by the drinking water quality standards in Japan (Kinoshita et al. 2005; Shibata et al. 2005). A total of 157 residents who were exposed to DPAA presented with progressive cerebellar and brain stem symptoms, including nystagmus, dizziness, ataxic gait, tremors, myoclonus and dysarthria, along with temporal and occipital lobe symptoms, including memory impairment, sleep disturbance, and visual disorders, as well as cerebral atrophy and mental retardation in children. The patients have been receiving regular medical checkups for more than 10 years.
Although mounting evidence strongly suggests that these neurological symptoms were caused by DPAA (Ishii et al. 2004), our knowledge about the in vivo distribution and effects of DPAA remains limited. The effects of DPAA in higher vertebrates have been demonstrated using rodents. The oral administration of DPAA to mice led to higher accumulation of DPAA in the brain than in the liver (Ozone et al. 2010). Furthermore, DPAA has been reported to damage the Purkinje cells and cause behavioral impairment in mice (Ozone et al. 2010; Umezu et al. 2012). In rats, the oral administration of DPAA led to a higher accumulation of DPAA in the brain and pancreas in comparison to inorganic arsenic compounds (Naranmandura et al. 2009), as well as behavioral impairment (Negishi et al. 2013). We previously administered DPAA to rats and established a rodent model with the same acute cerebellar and brain stem symptoms as those of patients in Kamisu (Masuda and Ishii, unpublished observation). However, their symptoms lasted for a shorter period compared to those of the patients (Masuda and Ishii, unpublished observation). Several studies have also shown that the distribution and metabolism of arsenic compounds are different among rodents and primates (Vahter and Marafante 1985; Vahter et al. 1995; Aposhian 1997). Therefore, it is difficult for us to predict the pattern of DPAA accumulation in the human central nervous system (CNS) solely based on the data from rodent studies. Thus, we decided to use primates to examine the accurate distribution of DPAA in the human CNS. The only primate study in relation to DPAA investigated the DPAA concentrations in the blood, urine, and feces of primates after oral administration (Kobayashi et al. 2008).
Previously, DPAA and its related metabolites have been identified in biological and environmental samples using liquid chromatography (LC) coupled with induced coupled plasma ionization mass spectrometry (ICP–MS), in which they were detected as arsenic atom-containing compounds (Kobayashi et al. 2008; Hempel et al. 2009). In the present study, we adopted isotope dilution LC–mass spectrometry (LC–MS). Internal standard compounds of DPAA and their metabolites labeled with 13C were added to the tissue samples. Unlabeled target phenyl arsenic compounds and their corresponding isotope-labeled congeners were extracted and subjected to LC–MS. Isotope dilution LC–MS is extremely useful because it can provide exact compensation of extraction recovery from biological samples and efficient mass spectrometric ionization.
In the present study, we used cynomolgus monkeys to predict the distribution and metabolism of DPAA in humans. We measured the concentration of DPAA in various tissue samples by LC–MS at 5–339 days after the last administration of DPAA.
Materials and methods
Arsenic
Phenyl arsenic compounds [DPAA (>99.0%), DPMAO (diphenylmethylarsine oxide: >99.0%), phenylmethylarsinic acid (PMAA: >99.0%), phenyldimethylarsine oxide (PDMAO: >99.0%), and phenylarsonic acid (PAA: >99.0%)] were purchased from Tri Chemical Laboratories (Yamanashi, Japan) and stored at 4 °C in the dark. Stable radioactive isotopes (13C-DPAA, 13C-DPMAO, 13C-PMAA, 13C-PDMAO, and 13C-PAA: >95.0%) were produced by Hayashi Pure Chemical Industries (Osaka, Japan).
Animals
Twenty-eight cynomolgus monkeys (Macaca fascicularis) were purchased from the HAMRI CO., LTD. (Ibaraki, Japan). The average weights of the male and female monkeys were 3.44 kg (n = 14) and 2.81 kg (n = 14), respectively. The administration of DPAA to the monkeys and tissue sampling were performed at LSI Medience Corporation (Tokyo, Japan). All of the experiments were conducted in accordance with the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan.
Animal experiments and the sampling of tissues and body fluids
DPAA was dissolved in distilled water at a concentration of 10 mg/mL and the pH was adjusted to 7.0 with 1 N NaOH. It was then administrated through a nasogastric tube at a dose of 1.0 mg/kg/day. This concentration was determined based on the approximate levels of human exposure in Kamisu. After the repeated administration of DPAA for 28 days, the monkeys were divided into four groups. Monkeys in the first group (n = 6), second group (n = 6), third group (n = 8) and fourth group (n = 8) were euthanized at 5, 29, 170, and 339 days after the last administration, respectively.
After their euthanization, the following tissue types were excised and collected: eight types derived from the CNS, the sciatic nerves, three types from the emunctories, eight types from the urogenital system, seven types from the digestive system, four types from the lymph system, four types from the exocrine system, three types from the endocrine system, two types from the respiratory system, two types from the muscular system, two types from the sense organs, and two types from the blood–vascular system (see Tables). These samples were then frozen in liquid nitrogen and stored at −80 °C. Also, four types of body fluids (cerebrospinal fluid, bile, hemocyte, and serum) were collected and stored at −80 °C.
Extraction of phenyl arsenic compounds
20% of the homogenates of tissue samples were prepared in 50 mM ammonium acetate solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan) using a Teflon homogenizer (Ikemoto Scientific Technology Co., Ltd., Tokyo, Japan). Then, these homogenized samples, as well as the cerebrospinal fluid (CSF) and bile samples were ultra-centrifuged at 105,000×g at 4 °C and the supernatants were obtained. The collected blood was centrifuged at 3000×g for 10 min and separated into hemocytes and serum. The hemocytes were washed three times with 50 mM of Tris-buffered saline (pH 7.4). Thereafter, 10 mM of Tris–HNO3 buffer was added to the hemocyte solution and centrifuged at 15,000×g for 30 min.
Next, 0.1 mL of bovine serum albumin (80 mg/mL; Takara Bio Inc., Shiga, Japan), 1 mL of 4 M NaOH, and 0.9 mL of H2O were added to 0.1 g of the samples prepared above. Then, stable radioactive isotopes 13C-DPAA, 13C-DPMAO, 13C-PMAA, 13C-PDMAO, and 13C-PAA (each 100 ppb) were added to the mixed solution and incubated at 90 °C for 3 h. After adding diethyl ether (Wako Pure Chemical Industries, Ltd.: >99.5%), the mixed solution was centrifuged for 5 min to extract phenyl arsenic compounds with diethyl ether. The diethyl ether was then removed from the phenyl arsenic compounds using a stream of dry N2. After the addition of nitric acid (Wako Pure Chemical Industries, Ltd.), the phenyl arsenic compounds were completely dissolved in 10 mL of H2O.
Analysis of phenyl arsenic
Shiseido Nanospace SI-2 (Shiseido Co., Ltd., Tokyo, Japan) and TSQ Quantum Ultra (Thermo Fisher Scientific Corp., CA, USA) LC–MS instruments were used. Chromatographic separation was achieved using two reversed-phase columns (Imtakt Unison UK-C18; 150 × 3.0 mm i.d., 3 µm thickness, Kyoto, Japan; Imtakt Unison UP-Phenyl, 150 × 3.0 mm i.d., 3 µm thickness) at 40 °C. 50 µL of sample solution was injected. In both columns, elution was performed using a linear gradient of 0.1% formic acid (Wako Pure Chemical Industries, Ltd.: 99.0%) in water [A] with 0.1% formic acid in methanol–water (1:9, v/v) [B] as follows: 95% [A]: 5% [B] (5-min hold), 95% [A]: 5% [B] to 100% [B] (5–10 min), 100% [B] (5-min hold), 100% [B] to 95% [A]: 5% [B] (20–10 min), 95% [A]: 5% [B] (14-min hold). The flow rate was maintained at 0.25 mL/min. Electrospray ionization was performed in positive ionization mode. The [M + H]+ions of the targeted compounds (DPAA, 13C-DPAA, DPMAO, 13C-DPMAO, PMAA, 13C-PMAA, PDMAO, 13C-PDMAO, PAA, 13C-PAA) were selected as the precursor ions. Selected reaction monitoring was used to quantify each compound. The precursor and product ions were monitored. The collision energies of the compounds were as follows: m/z 263 > 152, 30 eV for DPAA; m/z 275 > 158, 30 eV for 13C-DPAA; m/z 261 > 154, 35 eV for DPMAO; m/z 273 > 160, 35 eV for 13C-DPMAO; m/z 203 > 77, 21 eV for PAA; m/z 209 > 83, 21 eV for 13C-PAA; m/z 201 > 77, 35 eV for PMAA; m/z 207 > 83, 35 eV for 13C-PMAA; m/z 199 > 169, 25 eV for PDMAO; and m/z 205 > 175, 25 eV for 13C-PDMAO. To determine the compounds with greater precision, we monitored the other product ions as well: m/z 203 > 185 for PAA; m/z 201 > 94 for PMAA; m/z 199 > 77 for PDMAO. The MS parameters for the analysis were as follows: spray voltage, 4000 V; sheath gas (N2) pressure, 6 Pa; auxiliary gas (N2) pressure, 0.5 Pa; capillary temperature, 230 °C; vaporizing temperature, 450 °C; tube lens offset, 100 V; collision gas (Ar) pressure, 0.2 Pa. Data acquisition and instrument control were performed using the Xcalibur software program (Thermo Fisher Scientific Corp.).
Also, a phenyl column was used for the qualitative confirmation of the target compounds detected in the C18 column analysis, and to avoid the false-positive detection of the target compounds in the C18 column analysis. The retention times, peak widths at half height, and the limit of detection (LOD, signal-to-noise ratio = 3) of the phenyl arsenic compounds in aqueous solution on the C18 column analysis were 14.2 min, 0.086 min, and 14 pg/mL, respectively, for DPAA; 13.8 min, 0.066 min, and 6.2 pg/mL for DPMAO; 12.4 min, 0.086 min, and 140 pg/mL for PAA; 12.5 min, 0.065 min, and 130 pg/mL for PMAA; and 11.8 min, 0.097 min, and 62 pg/mL for PDMAO. Similarly, the values for the phenyl arsenic compounds that were run on the phenyl column were 14.3 min, 0.086 min, and 25 g/mL, respectively, for DPAA; 14.2 min, 0.063 min, and 18 pg/mL for DPMAO; 11.4 min, 0.233 min, and 910 pg/mL for PAA; 12.4 min, 0.106 min, and 83 pg/mL for PMAA; and 12.2 min, 0.064 min, and 130 pg/mL for PDMAO. The LOD values for the tissue extract samples were higher than those for aqueous solution, depending on the tissue sample matrix. This was due to interference from the matrix, which typically suppresses the ionization of the target compounds and prevents the detection of peaks with overlapping matrix components. In the C18 column analysis, the relative intra-day deviations (n = 4) of the peak area ratios on the product ion spectra for arsenic compounds (20 ng/mL) to those for corresponding 13C-labelled ones (5 ng/mL; internal standards) were 0.9, 1.8, 2.7, 5.5, and 3.5% for DPAA, DPMAO, PAA, PMAA and PDMAO, respectively. The relative intra-day deviations in the phenyl column analysis (n = 5) were 3.6, 1.3, 7.9, 4.2, and 3.7%, respectively.
Data analysis
To calculate the half-life (T 1/2; the time required for the concentration to decrease to half its initial value) of DPAA, we plotted the DPAA concentrations (at 5 and 29 days after the last administration of DPAA) in each of tissue and body fluid sample against the concentration values on the logarithmic scale. The half-lives were calculated based on the slope of the decay lines (slope = −k/2.303, T 1/2 = 0.693/k).
Statistical analyses and preparation of approximate graph curves
Tukey’s method was used for multiple group comparisons. The JMP software program (version 5.12-J, SAS Institute Inc., NC, USA) was used to perform the statistical analyses. P values of <0.05 were considered to indicate statistical significance. The JMP software program (version 5.12-J) was also used to perform a curvilinear regression analysis, which allowed us to approximate the quadratic curves.
Results
Liquid chromatography–mass spectrometry
DPAA and its related metabolites were identified in tissue samples by LC–MS using the isotope dilution method. These phenyl arsenic compounds were well separated in LC conditions using both C18 and phenyl columns. The [M + H]+ ions of the target compounds were carefully observed by electrospray ionization. 50 µL of the sample solution was injected into the LC–MS system to achieve optimal detection sensitivity. Imtakt Unison columns enabled large-volume injection without compromising the peak resolution. During selected reaction monitoring (SRM), the product ions and collision energies were selected by optimizing the conditions in the product ion scan spectra of the target precursor ions. A typical SRM chromatogram for the control phenyl arsenic compounds is shown in Fig. 1. Calibration curves were prepared for the peak area ratios of the target phenyl arsenic compounds of the corresponding 13C-labeled internal standards on the respective SRM chromatograms. We first used a C18 column to determine the target compounds in the samples. If positive peaks were observed on SRM, the presence of the corresponding product ion peaks was tested for PAA, PMAA, and PDMAO to provide definitive confirmation of their presence, because these highly hydrophilic compounds typically suffer from severe interference from the biological matrix components in sample extracts during LC separation and MS detection.
A selected reaction monitoring chromatogram for the phenyl arsenic compounds in aqueous solution. 50 µL of DPAA, DPMAO, PAA, PMAA, and PDMAO solution (each 33.3 ng/mL) was injected into the LC–MS system fitted with a C18 column. The 100% ionic intensities and mass transitions are shown on the right. (Color figure online)
Quantitative analysis of DPAA in the tissues of cynomolgus monkeys
Twenty-eight cynomolgus monkeys were exposed to DPAA for 28 days at a dose of 1.0 mg DPAA/kg/day. After the oral administration of DPAA, the monkeys were divided into four groups (each group contained 6–8 monkeys), which were euthanized at 5, 29, 170, and 339 days after the last administration of DPAA. The whole tissues and body fluids were collected after euthanasia (Table 1).
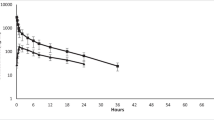
We measured the concentration of DPAA in the tissues of animals from each group using a LC–MS analysis. The concentrations of DPAA in all of the tissues showed a gradual decrease with time after the last administration (Table 2; Fig. 1). At 5 days after the last administration, we found that the average concentrations of DPAA in the tissues derived from the CNS (600 ± 21.2 ng As/g), the emunctories (1033 ± 188 ng As/g), and the sciatic nerve (480 ± 50.0 ng As/g) were significantly higher than those derived from other tissues (total 34 tissues, 43.2 ± 2.49 ng As/g) (Fig. 2; Table 1; p < 0.0001).
At 29 days after the last administration, the average concentrations of DPAA in the CNS tissues (181 ± 6.60 ng As/g) and the sciatic nerve (250 ± 23.9 ng As/g) were 30.1 and 52.1% of values at the 5-day time point, respectively (Fig. 3; Table 1). On the other hand, the average concentrations of DPAA in the emunctories (41.6 ± 8.32 ng As/g) and other tissues (4.76 ± 0.311 ng As/g) were drastically lower in comparison to those of the group that was euthanized at 5 days after the last administration (Fig. 3; Table 1) (4.03 and 11.0% of values at the 5-day time point, respectively). We also examined the half-life of DPAA in each tissue (Table 1). The average half-lives of DPAA in the CNS tissues and the sciatic nerve (14.4 and 25.7 days, respectively) were significantly longer in comparison to DPAA in other tissues (p < 0.0001).
Approximate curves showing the correlation between the days after the last administration of DPAA and the average concentrations of DPAA (ng As/g) in tissue samples from the CNS, the emunctories, the sciatic nerve, and other tissues of cynomolgus monkeys after the daily administration of DPAA (1.0 mg/kg/day) for 28 days. The average values ± SEM for each group are shown using horizontal lines. Purple, sciatic nerve; magenta, central nervous system; green, emunctories; blue, other tissues. *p < 0.0001, in comparison to the other tissues (the colors of the asterisks correspond to the group)
In the group that was euthanized at 339 days after the last administration, the concentrations of DPAA in all of the tissues were more than 96% lower than those in the group euthanized at 5 days after the last administration (Table 1). Notably, the average concentration of DPAA in the CNS tissues (18.8 ± 1.48 ng As/g) was significantly higher than that in all of the other tissues, even at 339 days after the last administration (Fig. 3; Table 1; p < 0.0001).
Quantitative analysis of DPAA in the body fluids of cynomolgus monkeys
Next, we measured the concentration of DPAA in the body fluids of each group using an LC–MS analysis. A small amount of DPAA was detected in the serum and the hemocytes at 5 and 29 days after the last administration (Fig. 4; Table 1). It was not detected after these time points. Similarly, DPAA was detected at a concentration of 0.233 ± 0.0268 ng As/g in the CSF at 29 days, and was not detected later (Fig. 4; Table 1). On the contrary, a large amount of DPAA was detected in the bile at 5 days after the last administration (2496 ± 287 ng As/g) (Table 1); the concentration at 29 days after the last administration was 3.79% of the value at the 5-day time point (94.5 ± 27.4 ng As/g). The average half-life of DPAA in the bile was 4.88 days.
Approximate curves showing the correlation between the days after the last administration of DPAA and the average concentrations of DPAA (ng As/g) in the body fluids (cerebrospinal fluid, bile, hemocyte and serum) of cynomolgus monkeys after the daily administration of DPAA (1.0 mg/kg/day) for 28 days. Blue, bile; green, hemocyte; yellow, serum; magenta, cerebrospinal fluid
Quantitative analysis of PAA and PMAA in cynomolgus monkeys
PAA and PMAA are known to be the major metabolites of DPAA. We measured the concentrations of PAA and PMAA in the same tissues of each group described above using an LC–MS analysis. We detected very small amounts of PAA and PMAA in these tissues at 5–339 days after the last administration (Tables 2, 3). The concentrations of PAA and PMAA in the CNS tissues (2.77 ± 0.522 and 0.973 ± 0.184 ng As/g, respectively) and emunctories (12.8 ± 4.36 and 1.66 ± 0.658 ng As/g, respectively) were a little higher than those in other tissues at 5 days after the last administration (Tables 2, 3).
Discussion
In the present study, we examined the clearance of orally administrated DPAA in cynomolgus monkeys. Our results clearly demonstrated that the half-life of DPAA in the brain was twice as long as that in other tissues except the emunctories, suggesting that DPAA easily accumulates in the brains of cynomolgus monkeys. Together with our preliminary observation that the half-life of DPAA in the rat brain after the administration of DPAA (at the same dose as this study) was shorter than that of cynomolgus monkeys (<8.6 days; Masuda and Ishii, unpublished observation), the findings suggest that DPAA may accumulate in the brains of primates more easily than in the rodent brain. Previous experiments using rodents have shown that inorganic arsenic (iAs) can cross the blood–brain barrier (BBB; Rodríguez et al. 2005; Juárez-Reyes et al. 2009). Similarly to organic arsenic, DPAA was also detected in the CSF obtained from the residents of Kamisu who were exposed to DPAA (Ishii et al. 2014). Their CSF/serum ratios of DPAA were 3.7 and 3.0% at the first and 14th days after their last exposure, respectively (Ishii et al. 2014). Consistent with these previous results, DPAA was detected in the CSF of cynomolgus monkeys in this study. The average CSF/serum ratio of DPAA in this study was 15.7% (5 days after the last administration, n = 6). Thus, these results strongly suggest that DPAA may pass through the BBB of primates. This may be due to the hydrophobicity of DPAA (Weksler et al. 2005; Naranmandura et al. 2009). Also, the reduction in blood flow and the cerebral metabolism was observed in the brains of the Kamisu residents who were exposed to DPAA (unpublished data). This evidence strongly suggests that the long presence of DPAA in the human brain may affect the brain function.
Several previous studies have shown that small amounts of iAs, monomethylarsenous acid (MMA) and dimethylarsinic acid (DMA) accumulated in the rat brain after the administration of iAs (Rodríguez et al. 2005, 2010; Juárez-Reyes et al. 2009). In humans, keratinization and pigmentation of the skin, peripheral neuropathy, skin cancer, and peripheral circulatory failure have all been reported as symptoms of chronic poisoning from exposure to inorganic arsenic compounds; however, there have been few reports on CNS symptoms (World Health Organization 2001). Previous rodent studies have revealed that DPAA, an organic arsenic compound, accumulated in the rodent brain more easily than inorganic arsenic compounds (Naranmandura et al. 2009; Ozone et al. 2010). Our study also demonstrated that DPAA accumulated in the CNS tissues of primates for a long period after its oral administration. These facts are consistent with our previous observations that the effects of DPAA on humans were substantially restricted to regions of the CNS such as the cerebellum and brain stem (Ishii et al. 2004). Furthermore, the cerebellar dysfunction found in the recent animal behavior experiments was quite similar to that observed in the Kamisu residents who were exposed to DPAA (Ozone et al. 2010; Negishi et al. 2013). Thus, it is highly likely that the effects of DPAA exposure on the CNS of Kamisu residents were caused by its long-term accumulation in the CNS.
It is known that when DPAA is administered to rats, it is excreted in the urine, feces, and hair (Naranmandura et al. 2009; Kobayashi and Hirano 2013). Although a high level of DPAA accumulation was seen in the liver, gallbladder, and bile of cynomolgus monkeys at 5 days after the last DPAA administration, the half-lives of the DPAA in these tissues were as short as those in other tissues. Thus, as is the case with rats, DPAA is likely to be excreted in the liver, gallbladder, and bile of cynomolgus monkeys. While the concentration of DPAA in rat hair at 14 days after the last DPAA administration (at the same dose as this study) was high (Masuda and Ishii, unpublished observation), moderate levels of DPAA and its metabolites were also detected in the skin with hair of cynomolgus monkeys at 5 days after the last DPAA administration in the present study. Thus, like the rat, it is highly likely that DPAA and its metabolites may be excreted in skin with hair in cynomolgus monkeys.
In summary, we showed, for the first time, that orally administered DPAA accumulated in the CNS tissues of primates. This finding could contribute to future studies to investigate the dynamics and metabolism of DPAA in humans.
References
Aposhian HV (1997) Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol 37:397–419. doi:10.1146/annurev.pharmtox.37.1.397
Hempel M, Daus B, Vogt C, Weiss H (2009) Natural attenuation potential of phenylarsenicals in anoxic groundwaters. Environ Sci Technol 43:6989–6995
Ishii K, Tamaoka A, Otsuka F, Iwasaki N, Shin K, Matsui A, Endo G, Kumagai Y, Ishii T, Shoji S, Ogata T, Ishizaki M, Doi M, Shimojo N (2004) Diphenylarsinic acid poisoning from chemical weapons in Kamisu, Japan. Ann Neurol 56:741–745. doi:10.1002/ana.20290
Ishii K, Itoh Y, Iwasaki N, Shibata Y, Tamaoka A (2014) Detection of diphenylarsinic acid and its derivatives in human serum and cerebrospinal fluid. Clin Chim Acta 431:227–231. doi:10.1016/j.cca.2014.01.029
Juárez-Reyes A, Jiménez-Capdeville ME, Delgado JM, Ortiz-Pérez D (2009) Time course of arsenic species in the brain and liver of mice after oral administration of arsenate. Arch Toxicol 83:557–563. doi:10.1007/s00204-008-0378-7
Kinoshita K, Shida Y, Sakuma C, Ishizaki M, Kiso K, Shikino O, Ito H, Morita M, Ochi T, Kaise T (2005) Determination of diphenylarsinic acid and phenylarsonic acid, the degradation products of organoarsenic chemical warfare agents, in well water by HPLC-ICP-MS. Appl Organomet Chem 19:287–293. doi:10.1002/aoc.789
Kobayashi Y, Hirano S (2013) The role of glutathione in the metabolism of diphenylarsinic acid in rats. Metallomics 5:469–478. doi:10.1039/C2MT20228J
Kobayashi Y, Negishi T, Mizumura A, Watanabe T, Hirano S (2008) Distribution and excretion of arsenic in cynomolgus monkey following repeated administration of diphenylarsinic acid. Arch Toxicol 82:553–561. doi:10.1007/s00204-007-0270-x
Kurata H (1980) Lessons learned from the destruction of the chemical weapons of the Japanese Imperial Forces. In: Chemical weapons: destruction and conversion. Stockholm International Peace Research Institute, London, pp 77–93
Naranmandura H, Suzuki N, Takano J, McKnight-Whitford T, Ogra Y, Suzuki KT, Le XC (2009) Systemic distribution and speciation of diphenylarsinic acid fed to rats. Toxicol Appl Pharmacol 237:214–220. doi:10.1016/j.taap.2009.03.023
Negishi T, Matsunaga Y, Kobayashi Y, Hirano S, Tashiro T (2013) Developmental subchronic exposure to diphenylarsinic acid induced increased exploratory behavior, impaired learning behavior, and decreased cerebellar glutathione concentration in rats. Toxicol Sci 136:478–486. doi:10.1093/toxsci/kft200
Ozone K, Ueno S, Ishizaki M, Hayashi O (2010) Toxicity and oxidative stress induced by organic arsenical diphenylarsinic acid and inorganic arsenicals and their effects on spatial learning ability in mice. J Health Sci 56:517–526. doi:10.1248/jhs.56.517
Rodríguez VM, Del Razo LM, Limón-Pacheco JH, Giordano M, Sánchez-Peña LC, Uribe-Querol E, Gutiérrez-Ospina G, Gonsebatt ME (2005) Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol Sci 84:157–166. doi:10.1093/toxsci/kfi057
Rodríguez VM, Limón-Pacheco JH, Carrizales L, Mendoza-Trejo MS, Giordano M (2010) Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol Teratol 32:640–647. doi:10.1016/j.ntt.2010.07.005
Shibata Y, Tsuzuku K, Komori S, Umedzu C, Imai H, Morita M (2005) Analysis of diphenylarsinic acid in human and environmental samples by HPLC-ICP-MS. Appl Organomet Chem 19:276–281. doi:10.1002/aoc.792
Umezu T, Nakamiya K, Kita K, Ochi T, Shibata Y, Morita M (2012) Diphenylarsinic acid produces behavioral effects in mice relevant to symptoms observed in citizens who ingested polluted well water. Neurotoxicol Teratol 34:143–151. doi:10.1016/j.ntt.2011.08.007
Vahter M, Marafante E (1985) Reduction and binding of arsenate in marmoset monkeys. Arch Toxicol 57:119–124
Vahter M, Couch R, Nermell B, Nilsson R (1995) Lack of methylation of inorganic arsenic in the chimpanzee. Toxicol Appl Pharmacol 133:262–268. doi:10.1006/taap.1995.1150
Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO (2005) Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 19:1872–1874. doi:10.1096/fj.04-3458fje
World Health Organization (2001) International Programme on Chemical Safety, Environmental Health Criteria 224: Arsenic and arsenic compounds, 2nd ed. Geneva. http://whqlibdoc.who.int/ehc/WHO_EHC_224.pdf. Accessed 14 Sept 2016
Acknowledgements
This work was supported by JSPS KAKENHI Grant Nos. JP20390039, JP16590811 and JP15K08148 from the program Grants-in-Aid for Scientific Research of the MEXT, Japan, and a Grant-in-Aid for research on Study (toxic study group) of the Health Effects of DPAA supported by the Ministry of the Environment.
Authors’ contributions
KI and AT designed the research; YS, TH, RT, TN, NI, YS, and TM performed the research; TM and KI analyzed the data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Masuda, T., Ishii, K., Seto, Y. et al. Long-term accumulation of diphenylarsinic acid in the central nervous system of cynomolgus monkeys. Arch Toxicol 91, 2799–2812 (2017). https://doi.org/10.1007/s00204-016-1928-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1928-z