Abstract
Aims/hypothesis
The ATP-regulated potassium (KATP) channel in the pancreatic beta cell couples the metabolic state to electrical activity. The primary regulator of the KATP channel is generally accepted to be changes in ATP/ADP ratio, where ATP inhibits and ADP activates channel activity. Recently, we showed that long-chain CoA (LC-CoA) esters form a new class of potent KATP channel activators in rodents, as studied in inside-out patches.
Methods
In this study we have investigated the effects of LC-CoA esters in human pancreatic beta cells using the inside-out and whole-cell configurations of the patch clamp technique.
Results
Human KATP channels were potently activated by acyl-CoA esters with a chain length exceeding 12 carbons. Activation by LC-CoA esters did not require the presence of Mg2+ or adenine nucleotides. A detailed characterization of the concentration-dependent relationship showed an EC 50 of 0.7±0.1 µmol/l. Furthermore, in the presence of an ATP/ADP ratio of 10 (1.1 mmol/l total adenine nucleotides), whole-cell KATP channel currents increased approximately six-fold following addition of 1 µmol/l LC-CoA ester. The presence of 1 µmol/l LC-CoA in the recording pipette solution increased beta-cell input conductance, from 0.5±0.2 nS to 2.5±1.3 nS.
Conclusion/interpretation
Taken together, these results show that LC-CoA esters are potent activators of the KATP channel in human pancreatic beta cells. The fact that LC-CoA esters also stimulate KATP channel activity recorded in the whole-cell configuration, points to the ability of these compounds to have an important modulatory role of human beta-cell electrical activity under both physiological and pathophysiological conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
ATP-sensitive potassium (KATP) channels play a key role in the coupling between cellular metabolism and electrical activity in a wide range of tissues. Since the discovery of these channels in the beta cell [1], their essential function has been included in the model of glucose-induced insulin secretion. In this model, increases in blood glucose levels lead to metabolism of glucose which generates an increase in the ATP/ADP ratio. The consensus view is that increases in the ATP/ADP ratio promote closure of the beta-cell KATP channel, thereby depolarizing the cell membrane resulting in opening of voltage-dependent Ca2+ channels. The subsequent rise in cytoplasmic free Ca2+ concentration triggers a series of events leading to exocytosis of insulin. Hence, the KATP channel provides a critical link between glucose metabolism, electrical activity and insulin secretion in the pancreatic beta cell.
The most important molecular regulators of KATP channel activity are believed to be adenine nucleotides, where intracellular ATP plays a central role. It has been shown that ATP inhibits KATP channel activity in excised membrane patches, with reported half-maximal inhibitory concentrations between 5 and 15 µmol/l [1, 2, 3]. Because of the presence of ATP in the millimolar range in the intact beta cell, endogenous agents capable of modulating ATP-sensitivity of the KATP channel must be of importance in the regulation of the channel. Several nucleotides, e.g. ADP and GDP, are known to stimulate KATP channel activity in the presence of inhibitory concentrations of ATP [4, 5]. Most studies have concentrated on the effects of ADP since the concentrations of ATP and ADP change reciprocally in response to glucose metabolism [6]. In the intact beta cell the ratio of ATP/ADP is considered more important than ATP alone in the regulation of KATP channel activity and thereby in the control of beta-cell membrane potential. Thus, substances with the ability to stimulate KATP channel activity are of special importance in the control of beta-cell membrane potential, electrical activity and thereby insulin secretion. Detailed characterization of endogenous substances with the ability to activate the channel is a fundamentally important area of diabetes research, especially in light of one report showing that over-activity of the beta-cell KATP channel induces profound neonatal diabetes [7]. These data indicate that reduction of the sensitivity of the channel to nucleotide inhibition can have a dramatic effect on insulin secretion, consistent with a crucial requirement for adequate control of KATP channel activity in normal regulation of insulin release.
In a series of publications, we have identified and characterized long chain acyl-coenzyme A (LC-CoA) esters as being a new class of molecules exhibiting a potent stimulatory effect on rodent beta-cell KATP channel activity [8, 9]. The LC-CoA esters, endogenous substances found in many cells including the pancreatic beta cell [10], were shown to be the most potent endogenous activators of the KATP channel, and able to reduce the sensitivity of the channel to ATP [8, 9]. The stimulatory effect seems to be confined to long-chain acyl-CoA esters, since addition of malonyl-CoA (3 carbon chain length) is without effect on the KATP channel [8]. Unlike another recently described group of lipid activators, anionic phospholipids [11, 12], LC-CoA esters seem to be highly specific for the KATP channel [8, 13]. The LC-CoA esters are, on a molar basis, 100 to 1000 times more potent than ADP [9] and both saturated and unsaturated LC-CoA esters induce a rapid, reversible activation of the beta-cell KATP channel [8]. In this study, we have characterized the effects of LC-CoA esters on the human pancreatic beta-cell KATP channel.
Materials and methods
Preparation of betacells
All pancreases were retrieved from brain dead cadaver heart-beating multiorgan-donors within the Eurotransplant area. After getting informed consent of one of the next relatives of the diseased, donor centers reported a potential multiorgan donor to Eurotransplant, Leiden, Netherlands. Organ allocation was done by Eurotransplant according to current guidelines of the donor country. For Germany, allocation was done according to the official guidelines in organ transplantation of the Bundesärztekammer. Islet preparations were used for research purpose only if they could not be used for clinical islet transplantation. The islets were isolated at Giessen Islet Isolation and Transplantation Center according to a modified semi-automated digestion-filtration method, followed by purification on a continuous density gradient on a refrigerated COBE 2991 centrifuge [14, 15]. Subsequently, islet preparations were placed in untreated culture flasks and maintained in suspension culture at 24°C (5% CO2 in humidified air) for 4 to 7 days. Two media changes were carried during that period. Islet volume and purity were determined by microscopical sizing on a grid after staining with diphenylthiocarbazone [16] and viability was assessed by membrane integrity testing (trypan blue exclusion). Islets were shipped to the department of Molecular Medicine at Karolinska Institutet, Stockholm, Sweden, where a cell suspension was prepared [17]. The dispersed beta cells were seeded into Petri dishes (Nunc, Roskilde, Denmark) and incubated at 37°C under 5% CO2 using RPMI 1640 culture medium (Flow Laboratories, Irvine, UK), supplemented with 10% foetal bovine calf serum and antibiotics (100 IU/ml pencillin, 100 µg/ml streptomycin and 60 µg/ml gentamycin). The study was performed in accordance with the declaration of Helsinki and the study was approved by the medical ethics committee at Huddinge University Hospital (reference ID 120/97).
Electrophysiology
KATP channel activity was recorded with the patch-clamp technique [18], using an Axopatch 200 patch-clamp amplifier (Axon Instruments, Foster City, Calif., USA). The recorded signal was stored on magnetic tape (JVC, Tokyo, Japan) at an upper cut-off frequency of 2 kHz. Single channel current traces were displayed according to the convention, upward deflections denoting outward currents. All experiments were carried out at room temperature (20–24°C) and channel activity was measured at 0 mV unless otherwise indicated. Whole cell KATP channel currents were recorded using the conventional or perforated-patch whole-cell configuration of the patch-clamp technique.
Solutions
The standard extracellular solution contained (in mmol/l): 138 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2 and 5 HEPES-NaOH at pH 7.4. For inside-out recordings, an intracellular-like solution (i.e. bath solution) consisted of (in mmol/l): 125 KCl, 1 MgCl2, 10 EGTA, 25 KOH and 5 HEPES-KOH at pH 7.15. In the perforated-patch experiments, the pipette solution contained (in mmol/l): 10 KCl, 76 K2SO4, 10 NaCl, 1 MgCl2, 10 HEPES-NaOH (pH 7.35) and 200 µg/ml of amphotericin B (dissolved in DMSO). The final concentration of DMSO was less than 0.1%. ATP was added as Mg2+ salt to the intracellular solution as shown in text and figures. Cis-9-monounsaturated oleoyl-CoA (C18:1), palmitoyl-CoA (C16:0), myristoyl-CoA (C14:0), lauroyl-CoA (C12:0), octanoyl-CoA (C8:0) and malonyl-CoA (C3:0) were prepared as stock solutions in deionized water (Millipore) and then added to the intracellular solution at the final concentration indicated in figures. All chemicals were of analytical grade and obtained from Sigma (St Louis, Mo. USA).
Data analysis
For analysis of mean current, channel recordings were filtered at 0.2 kHz (−3 dB value, 8 pole Bessel filter; Frequency Devices, Haverhill, Mass., USA), digitized at 0.8 kHz and stored in a computer, using an Axon Instrument analogue digital converter (TL-1). The mean current was calculated as previously described [5]. Data obtained from the concentration-dependence curves for LC-CoA esters were fitted to the Hill-equation:
where EC 50 is the concentration of LC-CoA esters ([LC-CoA]) causing half-maximal activation, h denotes the Hill value and L normalized maximal channel current. Data are shown as means±SE. Channel activity was compared using Student’s t-test or ANOVA for multiple groups and p values less than 0.05 were considered statistically significant. All experiments were repeated three times with similar results if not stated otherwise.
Results
Long-chain CoA esters stimulate KATP channel activity in the human pancreatic beta cell
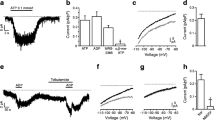
KATP channel activity was recorded in freshly excised patches from human pancreatic beta cells (Fig. 1). Addition of 1 µmol/l palmitoyl-CoA increased KATP channel activity (Fig. 1a). In contrast to other known KATP channel activators, such as ADP and diazoxide, which are ineffective in the absence of Mg2+, palmitoyl-CoA activated KATP channel activity in a non Mg2+-dependent manner (Fig. 1b). Furthermore, even in the presence of a high concentration of ATP (1 mmol/l) that fully blocks channel activity, addition of palmitoyl-CoA in the continuous presence of ATP was still able to activate the channel (Fig. 1c).
Effects of LC-CoA esters on the human pancreatic beta cell KATP channel. a, typical effect of 1 µmol/l palmitoyl-CoA on KATP channel activity recorded from a freshly excised inside-out patch obtained from a human pancreatic beta cell. b, stimulatory effects of 1 µmol/l palmitoyl-CoA observed in the absence of Mg2+. c, KATP channel activation induced by palmitoyl-CoA in the continued presence of 1 mmol/l ATP. d, effects of palmitoyl-CoA in the combined presence of 0.1 mmol/l ATP and 0.1 mmol/l diazoxide. e, ability of palmitoyl-CoA to reverse the inhibitory effect of 0.1 mmol/l tolbutamide
Administration of 0.1 mmol/l diazoxide in the presence of 0.1 mmol/l ATP counteracted the blocking effect of ATP. Inclusion of 1 µmol/l palmitoyl-CoA, in the continued presence of ATP and diazoxide, induced a marked further increase in KATP channel activity (Fig. 1d). In addition to being able to counteract the blocking effect of ATP, palmitoyl-CoA was also able to reverse the inhibitory effect of 0.1 mmol/l of the sulfonylurea tolbutamide (Fig. 1e).
KATP channel activation and chain-length dependence of acyl-CoA esters
To define the dependency of chain-length for the stimulatory action of acyl-CoA esters in human pancreatic beta cells, we tested the effects of esters with chain lengths between eight and eighteen on KATP channel activity. Only acyl-CoA esters with a chain length of 14 carbons (lauroyl-CoA) or longer gave rise to increased KATP channel currents (Fig. 2). There was a maximal effect obtained administering palmitoyl-CoA (C16:0), with no further increase following addition of oleoyl-CoA (C18:1).
Chain-length dependency of LC-CoA esters to induce KATP channel activation. a–c, typical recordings from inside-out patches excised from human pancreatic beta cells, exposed to acyl-CoA esters of various chain-length. d, compiled data on fatty acyl chain-length versus KATP channel currents (n=4–6 of each). The activity in the absence of acyl-CoA esters was set to 100%. ‘C’ denotes control. ** and *** denote p<0.01 and p<0.001, respectively
Effects of LC-CoA esters on single KATP channel kinetics
Addition of 300 nmol/l palmitoyl-CoA induced channel openings of longer duration as compared to the control situation (Fig. 3a). A detailed analysis of channel open-time from the same recording revealed that in the absence of nucleotides and LC-CoA, the distribution of KATP channel open-time was best described by a single exponential with a time constant of 33 ms (Fig. 3b left panel). Addition of palmitoyl-CoA to the same patch resulted in the appearance of a second component with a time constant of 177 ms, where 54% was related to the slow component (Fig. 3B right panel).
Effects of LC-CoA on single channel kinetics and single channel amplitude. a, typical recording of KATP channel currents from a freshly excised patch in the absence (top trace) and presence (bottom trace) of 300 nmol/l palmitoyl-CoA. b, frequency versus lifetime histogram of channel openings. Under control conditions, the distribution of channel lifetimes could be described by a single exponential with a time constant τ of 33.6 ms. A total number of 218 events were analyzed. In the presence of palmitoyl-CoA, distribution was best described as the sum of two exponentials with τ1=19.8 ms and τ2=177.4 ms. A total number of 308 events were analyzed where 54% of the integrated events belonged to the slow component. c, KATP channel I-V relationships at potentials between −80 mV and +80 mV in the absence (open circles) and presence (filled circles) of 1 µmol/l palmitoyl-CoA. d, single KATP channel currents recorded at the indicated potentials in the presence and absence of 0.1 mol/l palmitoyl-CoA. Current traces were filtered at 0.2 kHz and digitized at 0.8 kHz
Effects of LC-CoA esters in the presence of adenine nucleotides
Addition of 1 mmol/l ATP and 0.1 mmol/l ADP resulted in a dramatic decrease in KATP channel activity (Fig. 4a). Upon addition of 10 nmol/l palmitoyl-CoA no effect on channel activity could be seen, whereas 100 nmol/l palmitoyl-CoA clearly increased KATP channel activity, an effect which was even more pronounced at 1 µmol/l of the ester. Compiled data show a concentration-dependent increase in KATP channel currents following increasing concentrations of palmitoyl-CoA (Fig. 4b). Concentration-response data were fitted using Hill-equation, and resulted in an EC 50 of 0.7±0.1 µmol/l, and a Hill-value (h) of 3.7 (n=4–6). To verify that this was not an effect specific to the excised patch configuration, we studied whole-cell currents. The cells were voltage-clamped at −70 mV, on top of which voltage excursions of 5 mV (200 ms) were superimposed at a rate of 0.5 Hz, a protocol reflecting KATP channel conductance in the beta cell [19, 20]. Whole-cell input conductance in the presence of 0.1 mmol/l ATP and 0.1 mmol/l ADP was found to be around 0.4 nS, which slightly decreased with time (Fig. 4c). In experiments where 1 µmol/l of palmitoyl-CoA was included in the recording pipette solution we observed a slow but steady increase in whole-cell input conductance (Fig. 4c). Compiled data show that there was a pronounced increase in input conductance in cells perifused with palmitoyl-CoA after 3 min, an effect that was even more pronounced after 6 min (Fig. 4d). On average, the presence of 1 µmol/l palmitoyl-CoA induced a four- to five-fold increase in input conductance which corresponds well with what was observed in the excised patch experiments (Fig. 4b).
Effects of LC-CoA ester in the presence of ATP and ADP. a, inside-out recording of KATP channel activity showing effects of increasing concentrations of palmitoyl-CoA in the continued presence 1 mmol/l ATP and 0.1 mmol/l ADP. b, compiled data from experiments carried out as in a. Channel activity in the presence of 1 mmol/l ATP and 0.1 mmol/l ADP was set to 100% (n=4–6). c, recording of input conductance using the conventional whole-cell configuration. The recording pipette was filled with intracellular-like solution containing 1 mmol/l ATP and 0.1 mmol/l ADP alone (filled circles, n=5) or including 1 µmol/l palmitoyl-CoA (open circles, n=6). d, compiled results from recordings carried out as in c. * and ** denote p<0.05 and p<0.01, respectively
The presence of 5 mmol/l glucose resulted in an input conductance of approximately 0.7 nS (Fig. 5a). Following addition of 5 µmol/l oleic acid to the perifusing buffer, input conductance dramatically increased to around 4.4 nS. The increase in conductance was biphasic and slowly returned toward control value with time. In another set of experiments, we first incubated the cells in the absence of glucose for at least 30 min, after which 5 mmol/l glucose was introduced. Exposure of the beta cell to glucose decreased input conductance from 1.1 to 0.4 nS. Addition of 5 µmol/l oleic acid, in the continued presence of glucose, reversed the glucose-induced decrease in input conductance to a higher level than was found in the absence of glucose (Fig. 5b).
Effects of oleic acid on whole-cell KATP currents. Human pancreatic beta cells were voltage-clamped at −70 mV using the perforated patch configuration. Voltage excursions of 5 mV (200 ms) were applied every 2 s. a and b, examples of the effects of 5 µmol/l oleic acid added to the perfusion buffer, in the continued presence of 5 mmol/l glucose
Discussion
This study shows that LC-CoA esters are potent modulators of KATP channel activity in human beta cells as we previously found in insulin-secreting cell lines (own unpublished findings), primary mouse pancreatic beta cells [8, 9] as well as in transiently expressed KATP channels in oocytes [13, 21]. The experiments also provide further evidence that LC-CoA esters have the ability to counteract ATP-induced closure of the KATP channel in vitro. The data thereby point to the possibility of this class of lipid KATP channel activators to be involved in the suppression of glucose-induced depolarization following exposure to high levels of lipids. It has been shown previously that the site of action to induce a stimulatory effect of ADP differs from that of LC-CoA esters [9, 13, 21]. Since diazoxide has been suggested to share, at least partly, the site of interaction with ADP [22], additional activation by LC-CoA in the presence of diazoxide further support the presence of a unique site of action for LC-CoA esters to induce KATP channel activation.
We have earlier shown that the stimulatory effect of LC-CoA esters seems to be specific for the KATP channel since no stimulation was observed of the KCa or the 8 pS K+ channels, both of which are present in the beta cell [8]. This specificity is further indicated by expression studies, showing that the stimulatory effect of the acyl-CoA esters is conferred to the pore-forming subunit Kir6.2 of the KATP channel [13, 21]. Furthermore, a related Kir channel, Kir1.1a, which shares about 45% sequence identity with Kir6.2, is if anything inhibited by the LC-CoA ester [13]. In our study we have also in detail investigated to what extent the stimulatory effect of acyl-CoA is chain-length dependent. We found that only acyl-CoA esters with a chain-length exceeding 12 carbons are active. This is important to note from the point of view that overnight incubation with NEFA induces an increase in the levels of LC-CoA esters with no change in total amount of acyl-CoA esters [8]. Since added NEFA can be rapidly taken up by the cell [23] and converted to a LC-CoA ester, we examined the effect of oleic acid (C18:1) on whole-cell KATP currents. These data are in agreement with a previous report on NEFA modulation of KATP channel activity in clonal beta cells [24]. The stimulatory effect of NEFA on whole-cell K+ currents are not likely to be caused by a direct effect of NEFA on KATP channel activity since the NEFA, if anything, inhibits KATP channel activity as studied in excised patches [9]. The data rather point to the possibility that exogenously added NEFA is transported into the cell and converted to LC-CoA, which in turn promotes KATP channel activity.
The fact that the beta-cell membrane potential is mainly determined by the activity of the KATP channel [19] together with reports showing that KATP channel activity is not regulated normally in diabetic animal models [25], point to an important link between diabetes and a disturbed KATP channel function. One possible explanation to this is a decreased metabolic activity in the diabetic state, affecting the ability of glucose to increase the ATP/ADP ratio. However, although the general consensus is that the KATP channel is mainly regulated by alterations in the ATP/ADP ratio, several findings point to the fact that other routes are involved. Mutations of the site where ADP interacts with SUR1 prevent the stimulatory effects of ADP on KATP channel activity in inside-out patches, whereas metabolic activation is not affected to the expected extent in whole-cell recordings [22, 26]. This suggests the involvement of other endogenous substances in the regulation of the KATP channel. It is therefore essential to characterize in detail endogenous compounds with the ability to activate the KATP channel. This is a fundamentally important issue to clarify since targeted over-activity of the beta-cell KATP channel induces profound neonatal diabetes [7]. Thus, endogenous compounds reducing the sensitivity of the channel to nucleotide inhibition have a dramatic effect on insulin secretion, showing a crucial requirement for adequate control of KATP channel activity in normal regulation of insulin release. One such group of endogenous compounds could well be LC-CoA esters.
Abbreviations
- KATP :
-
ATP sensitive potassium channel
- LC-CoA:
-
Long-chain Co-enzyme A ester
References
Cook DL, Hales CN (1984). Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311:271–273
Ashcroft FM, Kakei M, Kelly RP, Sutton R (1987) ATP-sensitive K+ channels in human isolated pancreatic B-cells. FEBS Lett 215:9–12
Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J (1995) Reconstitution of I KATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270:1166–1170
Ashcroft FM, Rorsman P (1990) ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochem Soc Trans 18:109–111
Larsson O, Ämmälä C, Bokvist K, Fredholm B, Rorsman P (1993) Stimulation of the KATP channel by ADP and diazoxide requires nucleotide hydrolysis in mouse pancreatic beta-cells. J Physiol 463:349–365
Erecinska M, Bryla J, Michalik M, Meglasson MD, Nelson D (1992). Energy metabolism in islets of Langerhans. Biochim Biophys Acta 1101:273–295
Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG (2000) Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell 100:645–654
Larsson O, Deeney JT, Bränström R, Berggren PO, Corkey BE (1996) Activation of the ATP-sensitive K+ channel by long chain acyl-CoA: a role in modulation of pancreatic β-cell glucose sensitivity. J Biol Chem 271:10623–10626
Bränström R, Corkey BE, Berggren PO, Larsson O (1997) Evidence for a unique long chain acyl-CoA ester binding site on the ATP-regulated potassium channel in mouse pancreatic beta cells. J Biol Chem 272:17390–17394
Prentki M, Corkey BE (1996) Are the β-cell signaling molecules malonyl-CoA and cytosolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM. Diabetes 45:273–283
Fan Z, Makielski JC (1997) Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem 272:5388–5395
Shyng SL, Nichols CG (1998) Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282:1138–1141
Gribble FM, Proks P, Corkey BE, Ashcroft FM (1998) Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J Biol Chem 273:26383–26387
Ricordi C, Lacy PE, Finke EH, Olack B, Scharp DW (1980) Automated method for isolation of human pancreatic islets. Diabetes 37:413–420
Brandhorst H, Brandhorst D, Brendel MD, Hering BJ, Bretzel RG (1998) Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant 7:489–495
Ricordi C et al. (1990) Islet isolation assessment in man and large animals. Acta Diabetol Latina 27:185–195
Lernmark A (1974) The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia 10:431–438
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100
Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54:87–143
Larsson O, Kindmark H, Bränström R, Fredholm B, Berggren PO (1996) Oscillations in KATP-channel actvity promotes oscillations in cytoplasmic free Ca2+ concentration in the pancreatic β cell. Proc Natl Acad Sci USA 93:5161–5165
Bränström R, Leibiger I, Leibiger B, Corkey BE, Berggren PO, Larsson O (1998) Long chain coenzyme A esters activate the pore-forming subunit (Kir6.2) of the ATP-regulated potassium channel. J Biol Chem 273:31395–31400
Gribble FM, Tucker SJ, Ashcroft FM (1997) The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J 16:1145–1152
Hamilton JA, Johanson RA, Corkey BE, Kamp F (2001) Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci 16:99–108
Müller M, Szewczyk A, De Weille JR, Lazdunski M (1992) ATP-sensitive K+ channels in insulinoma cells are activated by nonesterified fatty acids. Biochemistry 31:4656–4661
Tsuura Y, Ishida H, Okamoto Y, Kato S, Sakamoto K, Horie M, Ikeda H, Okada Y, Seino Y (1993) Glucose sensitivity of ATP-sensitive K+ channels is impaired in beta-cells of the GK rat. A new genetic model of NIDDM. Diabetes 42:1446–1453
Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272:1785–1787
Acknowledgements
This work was supported by grants from the Swedish Research Council (31X-14303, 03X-09890, 72X-00034), the Swedish Diabetes Association, Swedish Society of Medical Research, the Nordic Insulin Foundation Committee, Novo Nordisk Foundation, Funds of Karolinska Institutet, The Family Persson Foundation, Berth von Kantzows Foundation and National Institutes of Health (DK58508). The Human Islet Isolation and Transplant Center at Giessen University is supported by the Juvenile Diabetes Research Foundation International.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bränström, R., Aspinwall, C.A., Välimäki, S. et al. Long-Chain CoA esters activate human pancreatic beta-cell KATP channels: potential role in Type 2 diabetes. Diabetologia 47, 277–283 (2004). https://doi.org/10.1007/s00125-003-1299-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1299-x