Abstract
Aims/hypothesis
The aim of this study was to determine the effects of preconditioning on injury and expression of heat shock proteins 70 in diabetic rat hearts.
Methods
Diabetes was induced by an intraperitoneal injection of 65 mg kg−1 streptozotocin. Daily subcutaneous injection of 4 IU insulin started 2 weeks after streptozotocin treatment for 4 weeks. Rats were preconditioned by intravenous injection of 10 mg kg−1 U50,488H, a selective κ-opioid receptor agonist (U50,488H preconditioning). The effects of U50,488H preconditioning had previously been shown to be blocked by a selective κ-opioid receptor antagonist, nor-binaltorphimine. Twenty-four hours later, rats were subjected to 30 min of regional ischaemia by occlusion of the left coronary artery followed by 4 h of reperfusion. Infarct size was determined at the end of reperfusion. Stress-inducible and constitutive heat shock proteins 70 were analysed at the end of ischaemia and reperfusion by Western blotting.
Results
Myocardial infarcts induced by ischaemia and reperfusion were greater in diabetic rats. U50,488H preconditioning significantly reduced the infarct size and increased the expression of stress-inducible heat-shock protein 70 in normal rats. The effects of U50,488H preconditioning were abolished in streptozotocin-induced diabetic rats, but restored by insulin replacement.
Conclusion/interpretation
In addition to a greater susceptibility to ischaemic insults, the delayed cardioprotection of U50,488H preconditioning was lost, which could at least partly be due to impaired synthesis of stress-inducible heat-shock protein 70 in diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preconditioning with a kappa-opioid receptor (κ-OR) agonist, U50,488H confers delayed cardioprotection [20, 22, 24] as does preconditioning with metabolic inhibition (MI) or myocardial ischaemia [20, 22, 24]. Preconditioning with either U50,488H or MI also increases the expression of both the constitutive (HSC70) and stress-inducible (HSP70) heat-shock proteins 70 in the heart [24]. Blockade of HSP70 synthesis, but not HSC70, with selective antisenses abolishes the cardioprotective effects of preconditioning with either U50,488H or MI, indicating that HSP70 mediates the cardioprotection [24]. The cardioprotective effects of preconditioning are κ-OR mediated since the effects on both cardiac protection and expression of HSP 70 are blocked by a selective antagonist, nor-binaltorphimine (nor-BNI) [24]. In rats made diabetic by streptozotocin (STZ), heat stress, which confers cardioprotection in normal rats [6, 17], fails to do so [8]. On the other hand, the treatment still induces heat-shock protein in diabetic rats as it does in normal rat [8]. We determined the delayed cardioprotection and expression of HSP70 following pharmacological preconditioning with U50,488H (UP) in STZ-induced diabetic rats. We also determined the effects of UP in insulin-treated diabetic rats.
Materials and methods
Development and characteristics of diabetic rats
Male Sprague-Dawley rats weighing 250 to 300 g were supplied by the Laboratory Animal Unit, from The University of Hong Kong. All experiments were approved by the Committee on the Use of Live Animals in Teaching and Research of The University of Hong Kong. Diabetes was induced by a single peritoneal injection of a sodium citrate buffer (0.1 mol/l, pH 4.5) containing streptozotocin (STZ, 65 mg kg−1 body weight). The normal rats were injected with the same volume of buffer only. The rats were kept for 6 weeks. One week later rats started to exhibit polydipsia and polyurea. After 2 weeks urine glucose was measured with glucose test strips (Medi-Test Combi 9) to confirm the development of diabetes. The diabetic rats were randomly divided into two groups. One group of diabetic rats received subcutaneous injection of insulin (4 IU per day) for a final 4 weeks. The other group received saline injection only.
After 6 weeks, the normal, diabetic and insulin-treated diabetic rats were weighed and anaesthetised with 60 mg kg−1 of pentobarbital sodium for studies on cardioprotection and expression of HSPs 70.
Pharmacological preconditioning with U50,488H
U50,488H dissolved in saline was given intravenously to the rat. The dose of U50,488H chosen was 10 mg kg−1, which we previously showed to confer maximal delayed cardioprotection [2]. That study also showed that the protection was abolished by co-administration of nor-BNI, a κ-OR antagonist [2].
Experimental protocol
Six weeks after injection of STZ, each of the three types of rats were divided into two groups, the UP group receiving intravenous injection of U50,488H while the other group received saline as control. The rat was anaesthetised and the chest opened 24 h after UP. An interval of 24 h was chosen based on our previous observation that the cardioprotection conferred by UP peaked at that time [2, 22]. The heart was then subjected to regional ischaemia for 30 min followed by reperfusion for 4 h. Myocardial infarct was determined at the end of reperfusion while expression of the heat shock proteins HSP 70 and HSC 70 were determined at the ends of ischaemia and reperfusion (Fig. 1).
Experimental protocol. Rats were infused saline intravenously with or without U50,488H. They were anaesthetised and their chests opened 24 h later. The heart was then subjected to 30 min of coronary occlusion followed by 4-h reperfusion. Infarct size was measured at the end of reperfusion while expression of heat-shock proteins was measured at the ends of ischaemia and reperfusion. N, normal rat; D, diabetic rat; Di, insulin-treated rat; UP, U50,488H preconditioning
Myocardial ischaemia and reperfusion
Rats were anaesthetised with urethane (120 mg kg−1 i.p.). Tracheotomy was done and a polythene cannula connected to a rodent ventilator (model 683, Harvard Apparatus, Holliston, Mass., USA) to permit artificial ventilation with room air supplemented with O2 at a rate of 60 to 65 strokes per min, a stroke volume of 1.0 to 1.5 ml 100 g−1 and a positive end expiratory pressure of 0.5 to 1 cm H2O. This maintained PCO2 at 18 to 24 mmHg, PO2 at 100 to 130 mmHg, and pH at 7.4 units. A Lead-II electrocardiogram was monitored via subcutaneous stainless steel electrodes. ECG was recorded continuously with a computer using a PowerLab/4SD analog-to-digital converter (AD Instruments, Castle Hill, Australia). A left thoracectomy was done at the fifth intercostal space followed by pericardiotomy and adjustment of the left atria appendage to reveal the location of the left coronary artery. A fine silk ligature was passed below the left anterior descending coronary artery (LADCA) from the area immediately below the left atrial appendage to the right portion of the left ventricle. The ends of the suture were threaded through a propylene tube to form a snare. After stabilisation for 20 min, the coronary artery was occluded by pulling the ends of the suture taut and clamping the snare onto the pericardial surface with a haemostat. Coronary artery occlusion was verified by pericardial cyanosis and the typical electrocardiographs of ST segment elevation and increased R-wave amplitude. Reperfusion was achieved by releasing the ligature. Successful reperfusion was confirmed by visualising a pericardial hyperaemic response.
Measurement of ischaemic risk zone and infarct size
The ligature around the coronary artery was re-tightened at the end of the 4-h reperfusion. The heart was removed and weighed. Approximately 0.25% Evans blue (0.2 ml) was infused into the heart to determine the myocardial risk zone. Then the heart was frozen and cut into five or six transverse slices of equal thickness (2 mm) from the apex to the base. After removing the right ventricle and the connective tissue, the slices were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC) in pH 7.4 buffer for 15 min at 37°C. The slices were immersed in 10% formalin overnight. The areas of infarct (IS, TTC negative), area at risk zone (AAR, TTC stained) and left ventricular zone (LV) were determined using a computerised planimetry technique (Optimas 5.2 software, Optimas, Seattle, Wash., USA). The area at risk was expressed as a percentage of the left ventricle (AAR/LV). The infarct size was expressed as a percentage of the risk zone (IS/AAR).
Determination of heat shock proteins by western blot analysis
Western blot analysis was used to determine the expression of both HSP70 and HSC70. A small piece of myocardial tissue was minced with a razor blade and immediately placed in a tissue dunce homogeniser in 2 ml of lysis RIPA buffer solution (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with 10 mg ml−1 PMSF, 1 µg ml−1 aprotinin and 1 µg ml−1 leupeptin added at the time of use (Santa Cruz Biotechnology, Calif., USA). The samples were homogenised and centrifuged at 11,000 g for 10 min. Then the supernatant was quickly frozen at −70°C and stored for further analysis. The overall protein concentration was determined by the Bio-Rad protein assay based on the Bradfold dye-binding procedure using bovine serum albumin as standard. Equal total protein loads of 60 µg were loaded on the lanes of SDS-polyacrylamide gel and separated by electrophoresis in 5% acrylamide stacking gel and 12% acrylamide separating gel initially at 100 V for 2 h. The separated proteins were transferred electrophoretically from the gel onto nitrocellulose membrane (0.45 µm pore size, Hybond-C) at 100 V in 4°C for 1.5 h in a buffer containing 25 mmol/l Tris-base, 192 mmol/l glycine, and 20% methanol. After the membranes had been washed in buffer for 60 min at room temperature (TBS pH 7.4, containing 0.1% Tween-20 and 5% skimmed milk power) to block non-specific binding sites, they were probed at 4°C overnight with rat anti-HSC70 monoclonal antibody (SPA-815) or mouse anti-HSP70 monoclonal antibody (SPA-810) both at 1:2000. After washing for 20 min with TBS—0.1% Tween-20 solution, the membranes were then incubated for 1 h with a secondary antibody solution conjugated to horseradish peroxidase-conjugated rabbit anti-rat or rabbit anti-mouse, both at 1:2000 dilution. The secondary antibody solution was decanted, and the membranes were washed for 30 min with TBS—0.1% Tween-20 alone. The bands representing the proteins were visualised using an enhanced chemiluminescence detection system. The level of each of the proteins in each group was expressed as a percentage of that of the corresponding control separated in the same gel. This procedure eliminated false results due to errors during loading, separation of proteins in the gel, transfer of protein bands to membrane and experiment-to-experiment variations in measurement of band density.
Drugs and chemicals
Trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl]-benzeneacetamide (U50,488H), streptozotocin (STZ), 2,3,5-triphenyltetrazolium chloride (TTC), and Evans blue dye were purchased from Sigma Chemical, St. Louis, Mo., USA; Medi-Test (Combi 9) reagent strips for urinalysis from Macherey-Nagel (Postfach, Düren, Germany); glucose concentration measurement kit from Biosystems (Barcelona, Spain); insulin zinc suspension of Monotard HM from Novo Nordisk (Bafsvaerd, Denmark); Rainbow-coloured protein markers (RPN-756), Hybond-P (PVDF transfer membrane RPN303F), ECL (RPN-2106) and Kodak Biomax Light-1 film from Amersham Biosciences UK limited (Buckinghamshire, UK); the primary antibody of rat anti-HSC70 monoclonal antibody (SPA-815) and the mouse anti-HSP70 monoclonal antibody (SPA-810) from StressGen company (Victoria, BC Canada); secondary antibodies of peroxidase-conjugated rabbit anti-rat immunoglobulins-HRP and peroxidase-conjugated rabbit anti-mouse immunoglobulins-HRP from DAKO (Glostrup, Denmark); and the quantity one software for imaging and quantitative analysis of relative density of heat shock proteins from Bio-Rad Laboratories (Hercules, Calif., USA).
Statistical analysis
All values were expressed as mean ± SEM. Hyperglycaemia, body weight, heart weight and infarct size were analysed by one-way ANOVA followed by Turkey-Kramer multiple comparison tests. Two-tailed unpaired Student’s t test was used to test the difference in protein expression between two groups. A p value of less than 0.05 was considered statistically significant.
Results
General characteristics of experimental rats
The blood glucose concentration, body weight (BW), heart weight (HW) and ratio of heart weight to body weight (BW/HW) of the normal rats (N), STZ-treated diabetic rats (STZ-treated) and STZ-treated diabetic rats with insulin replacement (STZ+ I) are presented in Table 1.
The blood glucose concentration was increased fourfold in STZ-treated diabetic rats whereas the body weight and heart weight were significantly lower than that in normal rats. The heart-to-body weight ratio was slightly, but significantly greater than the control rats with 6 weeks of STZ injection. Together with the polydipsia, polyurea and glucosuria observed, the results showed that STZ treatment induced Type 1 diabetes mellitus.
Daily insulin treatment of diabetic rats for 4 weeks restored the blood glucose concentration, and body and heart weights to control levels. The heart-to-body weight ratio of insulin-treated diabetic rats was also restored to close to that of normal rats although the difference was still not statistically significant from that of diabetic rats.
Effects of UP on the infarct size after ischaemia and reperfusion
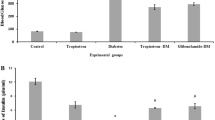
Myocardial risk size expressed as the percentage of the left ventricle was similar in all groups (Fig. 2a). However, myocardial infarct size induced by myocardial ischaemia and reperfusion was greater in STZ-treated diabetic rat (57.7±2.6%) than that in the control rats (41.5±2.4%,Fig. 2b). In insulin-treated diabetic rats the infarct size was 49.0±3.0%, which was between those of the control and diabetic rats without significant difference from either of them (Fig. 2b).
Effects of preconditioning with U50,488H (UP) on myocardial infarct induced by ischaemia and reperfusion in normal, STZ-induced diabetic and insulin-treated diabetic rats. (a) Risk at area (RAA) expressed as a percentage of the left ventricle (LV) and (b) Infarct size (IS) expressed as a percentage of risk zone (RA). N, saline in normal rats (n=11); N-UP, UP in normal rats (n=8); D, saline in diabetic rats (n=10); D-UP, UP in diabetic rats (n=11); and DI, saline in insulin-treated diabetic rats (n=8); DI-UP, UP in insulin-treated diabetic rats (n=7). Values are expressed as means ± SEM. *p<0.05 compared with N; # p<0.05 compared with DI
In normal rats, UP 24 h before ischaemic insult resulted in a reduction of infarct size from 41.5±2.4% to 28.2±3.2% (Fig. 2b). In contrast, in STZ-injected diabetic rats there was no significant difference on the infarct size between the diabetic rats with and without UP (49.7±3.0% vs 57.7±2.6%, Fig. 2b). In insulin-treated diabetic rats, UP also caused a reduction in infarct size from 49.0±3.0% to 34.9±2.5% (Fig. 2b), a response observed in the normal rats. The results showed that the cardioprotective effect of UP was abolished in diabetic rats, but restored by insulin replacement.
Effects of UP on the expression of HSP70 and HSC70
In normal rats the expression of HSP70 was significantly greater at the ends of ischaemia and reperfusion in normal rats with UP than in the control rats (Fig. 3a). On the other hand in STZ-treated diabetic rats there were no significant differences in the expression of HSP70 with and without UP (Fig. 3b). In STZ-treated rats with insulin replacement the expression of HSP70 was also greater at the ends of ischaemia and reperfusion in the group subjected to UP (Fig. 3c), a response similar to that in the control rats (Fig. 3a).
Effects of preconditioning with U50,488H (UP) on expression of stress-inducible heat-shock protein 70 (HSP70) in the heart of normal (a), STZ-induced diabetic (b), and insulin-treated diabetic (c) rats. Groups N, D and DI represent normal, diabetic and insulin-treated diabetic rats. Upper panel: Representative bands of HSP70, Lower panel: Group results. Values are presented as means ± SEM. n=5–7 per group. Density of proteins in UP-treated groups expressed relative to that of corresponding values of the respective control, which was arbitrarily given a value of 1.0. *p<0.05 vs corresponding control
In contrast there was no difference in expression of HSC70 among all three types of rats with and without UP (Fig. 4).
Effects of preconditioning with U50,488H (UP) on expression of constitutive heat-shock protein 70 (HSC 70) in the heart of normal (a), STZ-induced diabetic (b) and insulin-treated diabetic (c) rats. Groups N, D and DI represent normal, diabetic and insulin-treated diabetic rats. Upper panel: representative bands of HSC70, Lower panel: Group results. Values are presented as means ± SEM. n=5–7 per group. Density of proteins in UP-treated groups is expressed relative to that of corresponding values of the respective control, which is arbitrarily given a value of 1.0
Discussion
The novel observations of the present study are that in STZ-induced diabetes UP failed to confer delayed cardioprotection and to increase the synthesis of HSP70 in response to ischaemia and reperfusion and that insulin replacement restored these effects of UP, indicating that cardioprotection is lost and synthesis of HSP70 impaired after UP in Type 1 diabetes. Since HSP70 has been shown to mediate the delayed cardioprotection of UP, the finding indicates that impaired synthesis of HSP70 is responsible at least partly for its abolition.
One of the diabetic complications is susceptibility to myocardial ischaemic injury. We found that the myocardial infarct caused by ischaemia and reperfusion was significantly greater in diabetic than in normal rats in agreement with previous observations [3, 11, 14]. More importantly, we showed that UP failed to confer delayed cardioprotection, which is also in agreement with the previous observation that heat stress, which confers cardioprotection in normal rats [6], also fails to do so in diabetic rats [8]. So in diabetic rats, the injury in response to ischaemic insult is greater and the cardioprotection of preconditioning is lost. These are responsible for the greater vulnerability to ischaemic injury in diabetes.
In a previous study it was shown that in diabetic rats, heat stress enhances the expression of HSP70, but fails to confer delayed cardioprotection [8]. This suggests that failure to confer cardioprotection may not be due to a reduction in the synthesis of HSP70, but rather to a reduction in responsiveness to HSP70 in diabetic rats. Alternatively, HSP70 is not involved in cardioprotection of preconditioning with heat stress. We found that in diabetic rats UP not only failed to confer cardioprotection, but also failed to enhance the synthesis of HSP70, known to mediate delayed cardioprotection of UP [24]. The finding indicates that impaired synthesis of HSP70 is responsible at least partly for the failure to confer delayed cardioprotection of UP in diabetes. This is in agreement with previous findings. In Type 2 diabetes, the expression of HSP72 in skeletal muscle is decreased and is accompanied by impaired glucose metabolism [10]. The up-regulation of HSP70 upon cutaneous wounding is also delayed, which could be related to impaired inflammatory responses in diabetic mice [12]. It is interesting to note that we found that insulin deficiency due to destruction of the beta cells by STZ abolished the effect of UP on expression of HSPs, whereas insulin replacement restored this effect. The observation could suggest that insulin itself is directly responsible for effect of UP. In support, insulin has been shown to have a direct effect on κ-OR in rats [16]. On the other hand in Type 2 diabetes when insulin is present, the expression of HSP 72 in skeletal muscle is decreased, accompanied by impaired glucose uptake, and carbohydrate and lipid metabolism [12], secondary to attenuation or lack of response to insulin, but not lack of insulin. This suggests that effects of UP require normal glucose metabolism.
In a previous study we found that preconditioning with either U50,488H, a selective κ-OR agonist, or MI, confers delayed cardioprotection, which is accompanied by an increased expression of HSP70 and HSC70. Both the cardioprotection and the expression of HSP70 are attenuated by blockade of κ-OR with a selective κ-OR antagonist, nor-BNI, or inhibition of the synthesis of the HSP70 with a selective antisense [24]. The data provide evidence of a link between HSP and OR in cardioprotection. In support of our observation, heat shock treatment, that increases the expression of HSPs, confers cardioprotection and administration of naloxone, a non-selective OR antagonist, attenuates both the cardioprotective effect and increased expression of HSPs [15].
In a recent study we showed that KATP channels play an important role in delayed cardioprotection of UP [2]. There is also evidence that impaired KATP channels are responsible for failure to precondition the heart in diabetic people [4] and in sheep [19]. The impaired channels could be linked to the failure to enhance HSP expression in diabetes. However, it has been shown in the rabbit that heat stress confers cardioprotection and that blockade of the KATP channels with their blockers during ischaemia and reperfusion abolishes the effect of heat stress on cardioprotection, but not on HSP72 expression [5], indicating the cardioprotective action of HSP might not directly link to the channels. We have also shown that PKC-ε mediates delayed cardioprotection of UP [21]. Evidence on the relationship between PKC and HSP in cardioprotection is controversial. In the rat heart overexpression of HSP72 induced by geranylgeranylacetone (GGA), an antiulcer drug, confers cardioprotection against ischaemia and reperfusion and is accompanied by increased PKC activity. Blockade of PKC abolishes all the effects of GGA on expression of HSP72 and cardioprotection [23]. The result is evidence of a link between PKC and HSP in cardioprotection. However, it has also been shown that heat stress confers cardioprotection and enhances HSP72 expression in the rat and that blockade of PKC abolishes the effect on cardioprotection, but not on HSP expression, indicating a lack of a link between PKC and HSP in cardioprotection [7, 9]. Reactive oxygen species (ROS) also play an essential role in delayed cardioprotection of ischaemic preconditioning [18, 25]. There is evidence showing that generation of ROS by heat stress increases HSPs, which in turn renders cardioprotection [13]. There is also evidence that generation of ROS by heat stress renders cardioprotection independent of HSP production [1]. Further studies are needed to delineate the signalling mechanisms of cardioprotection of activation of HSP in health and diabetes.
In a previous study we showed that HSC70 expression was increased after ischaemic insults in ventricular myocytes preconditioned with U50,488H [24]. In the present study we failed to find a significant increase in the expression of the protein in the intact rat heart subjected to ischaemia and reperfusion following UP. The previous study used an in vitro model while the present study was carried out in vivo. The common finding in both studies is that HSC70 is not involved in delayed cardioprotection of UP.
UP has been shown to confer delayed cardioprotection in isolated ventricular myocytes [22]. In an earlier study we administered U50,488H intravenously and found that the infarct size in the isolated perfused heart in response to ischaemia and reperfusion is significantly reduced 24 h later compared with the control, indicating delayed cardioprotection in an in vivo preparation [2]. In the present study we also found a reduction in infarct size in response to ischaemia and reperfusion in the intact heart of anaesthetised rats subjected to UP 24 h earlier. This observation is another piece of evidence for delayed cardioprotection of UP in vivo.
One possible reason for the lack of enhanced HSP expression and cardioprotection after UP in diabetic rats could be that the dose of U50,488H was too low. However, a previous study from our laboratory [2] showed that the delayed cardioprotection is directly correlated to the dose in the range of 3 to 10 mg kg−1 with the maximal effect at 10 mg kg−1. A further increase in U50,488H does not confer any greater cardioprotection. It is therefore unlikely that a dose of U50,488H greater than 10 mg kg−1 would be able to confer cardioprotection and elicit an enhanced HSP expression in diabetic rats.
In conclusion, we have provided evidence showing that UP fails to confer delayed cardioprotection and to increase HSP70 synthesis in STZ-induced diabetic rats. Together with the previous finding that HSP70 mediates delayed cardioprotection of UP, this finding indicates that in diabetic rats, the failure to confer delayed cardioprotection results, at least partly, from impaired HSP70 production.
Abbreviations
- STZ:
-
Streptozotocin
- κ-OR:
-
kappa-opioid receptor
- U50,488H:
-
Trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl] benzeneacetamide
- UP:
-
U50,488H preconditioning
- LADCA:
-
left anterior descending coronary artery
- ECG:
-
electrocardiography
- TTC:
-
2,3,5-triphenyltetrazolium chloride
- HSP70:
-
stress-inducible heat-shock protein 70
- HSC70:
-
constitutive heat shock protein 70
References
Arnaud C, Joyeux M, Garrel C, Godin-Ribuot D, Demenge P, Ribout C (2002) Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br J Pharmacol 135:1776–1782
Chen M, Zhou JJ, Kam WL et al. (2003) Roles of KATP channels in delayed cardioprotection and intracellular Ca2+ homeostasis in the rat heart by stimulation of κ-opioid receptor with U50,488H. Br J Pharmacol 140:750–758
Forrat R, Sebbag L, Wiernsperger N et al. (1993) Acute myocardial infarction in dogs with experimental diabetes. Cardiovasc Res 27:1908–1912
Ghosh S, Standen NB, Galinanes M (2001) Failure to precondition pathological human myocardium. J Am Coll Cardiol 37:711–718
Hoag JB, Qian Y-Z, Nayheem MA, D’AngeloM, Kukreaja RC (1997) ATP-sensitive potassium channel mediates delayed ischemic protection by heat stress in rabbit heart. Am J Physiol Heart Circ Physiol 273:H2458–H2464
Hutter MM, Sievers RE, Barbosa V et al. (1994) Heat shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation 89:355–360
Joyeux M, Baxter GF, Thomas DL, Ribuot C, Yellon DM (1997) Protein kinase C is involved in resistance to myocardial infarction induced by heat stress. J Mol Cell Cardiol 29:3311–3319
Joyeux M, Faure P, Godin-Ribuot D et al. (1999) Heat stress fails to protect myocardium of streptozotocin-induced diabetic rats against infarction. Cardiovasc Res 43:939–946
Kukreja RC, Qian YZ, Okubo S, Flaherty EE (1999) Role of protein kinase C and 72 kDa heat shock protein in ischemic tolerance following heat stress in the rat heart. Mol Cell Biochem 195:123–131
Kurucz I, Morva A, Vaag A et al. (2002) Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51:1102–1109
Marfella R, D’Amico M, Filippo CD et al. (2002) Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 45:1172–1181
McMurtry AL, Cho K, Young LJT et al. (1999) Expression of HSP70 in healing wounds of diabetic and nondiabetic mice. J Surg Res 86:36–41
Nishizawa J, Nakai A, Matsuhiko M, Komeda M, Ban T, Nagata K (1999) Reactive oxygen species play an important role in the activation of heat shock factor 1 inischemic-reperfused heart. Circulation 99:934–941
Palik I, Koltai MZ, Kolonics I et al. (1982) Effects of coronary occlusion and norepinephrine on the myocardium of alloxan-diabetic dogs. Basic Res Cardiol 77:499–506
Patel HH, Hsu A, Gross GJ (2002) Attenuation of heat shock-induced cardioprotection by treatment with the opiate receptor antagonist naloxone. Am J Physiol Heart Circ Physiol 282:H2011–H2017
Sipols AJ, Bayer J, Bennett R Figlewicz DP (2002) Intraventricular insulin decreases kappa opioid receptor mediated sucrose intake in rats. Peptides 23:2181–2187
Steare SE, Yellon DM (1993) The protective effect of heat stress against reperfusion arrhythmias in the rat. J Mol Cell Cardiol 25:1471–1481
Sun JZ, Tang XL, Qiu Y, French BA, Bolli R (1996) Evidence for an essential role of reactive oxygen species in the gegesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest 97:562–576
Valle HF del, Lascano EC, Negroni JA (2002) Ischemic preconditioning protection against stunning in conscious diabetic sheep: role of glucose, insulin, sarcolemmal and metochondrial KATP channels. Cardiovasc Res 55:642–659
Wang GY, Wu S, Pei JM, Yu XC, Wong TM (2001) κ- but not δ-opioid receptors mediate effects of ischemic preconditioning on both infarct and arrhythmias in rats. Am J Physiol Heart Circ Physiol 280:H384–H391
Wang GY, Zhou JJ, Shan J, Wong TM (2001) PKC-ε is a trigger of delayed cardioprotection of κ-opioid receptor stimulation in the rat heart. J Pharmacol Exp Ther 299:603–610
Wu S, Li HY, Wong TM (1999) Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of kappa-opioid receptor. Circ Res 84:1388–1395
Yamanaka K, Takashi N, Ooie T, Kaneda K, Yoshimatsu H, Saikawa T (2003) Role of protein kinase C in geranylgeranylacetone-induced expression of heat shock protein 72 and cardioprotection in the rat heart. J Mol Cell Cardiol 35:785–794
Zhou JJ, Pei JM, Wang GY, Wu S, Wang WP, Cho CH, Wong TM (2001) Inducible HSP70 mediates delayed cardioprotection via U50,488H pretreatment in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 281:H40–H47
Zhou X, Zhai X, Ashraf M (1996) direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation 93:1177–1184
Acknowledgements
The study was supported by a Cardiovascular Physiology Research Fund donated by L.C.S.T. (Holding) Ltd. We thank Prof. F. Tang for advice; Dr. I. Bruce for reading the manuscript and Mr. C.P. Mok for assistance. Jian-Song Qi was on leave from China Pharmaceutical University, Nanjing, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, J.S., Kam, K.W.L., Chen, M. et al. Failure to confer cardioprotection and to increase the expression of heat-shock protein 70 by preconditioning with a κ-opioid receptor agonist during ischaemia and reperfusion in streptozotocin-induced diabetic rats. Diabetologia 47, 214–220 (2004). https://doi.org/10.1007/s00125-003-1288-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1288-0