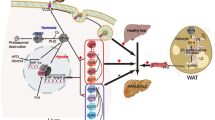
Abstract
The pandemic rise in obesity has resulted in an increased incidence of metabolic complications. Non-alcoholic fatty liver disease is the hepatic manifestation of the metabolic syndrome and has become the most common chronic liver disease in large parts of the world. The adipose tissue expansion and hepatic fat accumulation characteristics of these disorders compromise local oxygen homeostasis. The resultant tissue hypoxia induces adaptive responses to restore oxygenation and tissue metabolism and cell survival. Hypoxia-inducible factors (HIFs) function as master regulators of this hypoxia adaptive response, and are in turn hydroxylated by prolyl hydroxylases (PHDs). PHDs are the main cellular oxygen sensors and regulate HIF proteasomal degradation in an oxygen-dependent manner. HIFs and PHDs are implicated in numerous physiological and pathological conditions. Extensive research using genetic models has revealed that hypoxia signaling is also a key mechanism in adipose tissue dysfunction, leading to adipose tissue fibrosis, inflammation and insulin resistance. Moreover, hypoxia affects liver lipid metabolism and deranges hepatic lipid accumulation. This review summarizes the molecular mechanisms through which the hypoxia adaptive response affects adipocyte and hepatic metabolism, and the therapeutic possibilities of modulating HIFs and PHDs in obesity and fatty liver disease.


Similar content being viewed by others
References
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384(9945):766–781
Yki-Jarvinen H (2014) Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2(11):901–910
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2015) Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology. doi:10.1002/hep.28431
Byrne CD, Targher G (2015) NAFLD: a multisystem disease. J Hepatol 62(1 Suppl):S47–S64
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P (2015) Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 47(3):181–190
Semenza GL (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24:97–106
Suzuki T, Shinjo S, Arai T, Kanai M, Goda N (2014) Hypoxia and fatty liver. World J Gastroenterol 20(41):15087–15097
Nath B, Szabo G (2012) Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology 55(2):622–633
Palmer BF, Clegg DJ (2014) Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol 397(1–2):51–58
Ye J, Gao Z, Yin J, He Q (2007) Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293(4):E1118–E1128
Rausch ME, Weisberg S, Vardhana P, Tortoriello DV (2008) Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32(3):451–463
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867
Trayhurn P, Wood IS (2004) Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92(3):347–355
Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58(3):718–725
Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A (2004) Obesity decreases perioperative tissue oxygenation. Anesthesiology 100(2):274–280
Hodson L, Humphreys SM, Karpe F, Frayn KN (2013) Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes 62(5):1417–1425
Goossens GH, Bizzarri A, Venteclef N et al (2011) Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124(1):67–76
Chechi K, Nedergaard J, Richard D (2014) Brown adipose tissue as an anti-obesity tissue in humans. Obes Rev 15(2):92–106
Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K (2014) Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest 124(5):2099–2112
Samuel VT, Shulman GI (2016) The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 126(1):12–22
Sattar N, Gill JM (2014) Type 2 diabetes as a disease of ectopic fat? BMC Med 12:123
Jungermann K, Kietzmann T (2000) Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 31(2):255–260
Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG (1997) Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology 25(4):920–926
Arteel GE, Raleigh JA, Bradford BU, Thurman RG (1996) Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am J Physiol 271(3 Pt 1):G494–G500
Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM (2009) High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 417(1):183–193
Wang GL, Semenza GL (1993) Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem 268(29):21513–21518
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92(12):5510–5514
Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H (2011) Angiogenesis in chronic liver disease and its complications. Liver Int 31(2):146–162
Ichiki T, Sunagawa K (2014) Novel roles of hypoxia response system in glucose metabolism and obesity. Trends Cardiovasc Med 24(5):197–201
Kaelin WG Jr (2002) Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2(9):673–682
Keith B, Johnson RS, Simon MC (2012) HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12(1):9–22
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71
Loboda A, Jozkowicz A, Dulak J (2012) HIF-1 versus HIF-2–is one more important than the other? Vascul Pharmacol 56(5–6):245–251
Fraisl P, Mazzone M, Schmidt T, Carmeliet P (2009) Regulation of angiogenesis by oxygen and metabolism. Dev Cell 16(2):167–179
Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH (2006) Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 281(33):23482–23491
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279(37):38458–38465
Corvera S, Gealekman O (2014) Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 1842(3):463–472
Christiaens V, Lijnen HR (2010) Angiogenesis and development of adipose tissue. Mol Cell Endocrinol 318(1–2):2–9
Trayhurn P (2013) Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93(1):1–21
Lee YS, Kim JW, Osborne O et al (2014) Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157(6):1339–1352
Busiello RA, Savarese S, Lombardi A (2015) Mitochondrial uncoupling proteins and energy metabolism. Front Physiol 6:36
Trayhurn P, Alomar SY (2015) Oxygen deprivation and the cellular response to hypoxia in adipocytes–perspectives on white and brown adipose tissues in obesity. Front Endocrinol (Lausanne) 6:19
Halberg N, Khan T, Trujillo ME et al (2009) Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29(16):4467–4483
Hosogai N, Fukuhara A, Oshima K et al (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56(4):901–911
Huang LE, Gu J, Schau M, Bunn HF (1998) Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95(14):7987–7992
Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409(6821):729–733
Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ (2011) Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60(10):2484–2495
Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE (2013) Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol 33(5):904–917
Shimba S, Wada T, Hara S, Tezuka M (2004) EPAS1 promotes adipose differentiation in 3T3-L1 cells. J Biol Chem 279(39):40946–40953
Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB (2014) Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63(10):3359–3371
Garcia-Martin R, Alexaki VI, Qin N, et al (2015) Adipocyte-specific HIF2α deficiency exacerbates obesity-induced brown adipose tissue dysfunction and metabolic dysregulation. Mol Cell Biol 36(3):376–393
Lin Q, Huang Y, Booth CJ, Haase VH, Johnson RS, Celeste Simon M, Giordano FJ, Yun Z (2013) Activation of hypoxia-inducible factor-2 in adipocytes results in pathological cardiac hypertrophy. J Am Heart Assoc 2(6):e000548
Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR (2011) The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab 14(4):491–503
Wood IS, Stezhka T, Trayhurn P (2011) Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflugers Arch 462(3):469–477
Palmer BF, Clegg DJ (2014) Ascent to altitude as a weight loss method: the good and bad of hypoxia inducible factor activation. Obesity (Silver Spring) 22(2):311–317
Cushman SW, Wardzala LJ (1980) Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem 255(10):4758–4762
Selvaraju V, Parinandi NL, Adluri RS, Goldman JW, Hussain N, Sanchez JA, Maulik N (2014) Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases. Antioxid Redox Signal 20(16):2631–2665
Besarab A, Chernyavskaya E, Motylev I et al (2015) Roxadustat (FG-4592): correction of Anemia in Incident Dialysis Patients. J Am Soc Nephrol 27(4):1225–1233
Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, Leong R, Hemmerich S, Yu KH, Neff TB (2015) Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 30(10):1665–1673
Matsuura H, Ichiki T, Inoue E, Nomura M, Miyazaki R, Hashimoto T, Ikeda J, Takayanagi R, Fong GH, Sunagawa K (2013) Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127(21):2078–2087
Raguso CA, Guinot SL, Janssens JP, Kayser B, Pichard C (2004) Chronic hypoxia: common traits between chronic obstructive pulmonary disease and altitude. Curr Opin Clin Nutr Metab Care 7(4):411–417
Michailidou Z, Morton NM, Moreno Navarrete JM, West CC, Stewart KJ, Fernandez-Real JM, Schofield CJ, Seckl JR, Ratcliffe PJ (2015) Adipocyte pseudohypoxia suppresses lipolysis and facilitates benign adipose tissue expansion. Diabetes 64(3):733–745
Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG Jr (2009) A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol 29(21):5729–5741
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7(1):77–85
Wong BW, Kuchnio A, Bruning U, Carmeliet P (2013) Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci 38(1):3–11
He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J (2011) Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300(5):E877–E885
Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145(5):732–744
Taniguchi CM, Finger EC, Krieg AJ et al (2013) Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat Med 19(10):1325–1330
Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, Xu A (2010) Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem 285(43):32869–32877
Birkenfeld AL, Shulman GI (2014) Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59(2):713–723
Virtue S, Vidal-Puig A (2010) Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta 1801(3):338–349
Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE (2012) Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 109(15):5874–5879
Sung HK, Doh KO, Son JE et al (2013) Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17(1):61–72
Elias I, Franckhauser S, Ferre T et al (2012) Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61(7):1801–1813
During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, Cao L (2015) Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology 156(6):2059–2073
Lolmede K, de Saint Durand, Front V, Galitzky J, Lafontan M, Bouloumie A (2003) Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord 27(10):1187–1195
Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J (2009) Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296(2):E333–E342
Xiong Y, Qu Z, Chen N, Gong H, Song M, Chen X, Du J, Xu C (2014) The local corticotropin-releasing hormone receptor 2 signalling pathway partly mediates hypoxia-induced increases in lipolysis via the cAMP-protein kinase A signalling pathway in white adipose tissue. Mol Cell Endocrinol 392(1–2):106–114
Hashimoto T, Yokokawa T, Endo Y, Iwanaka N, Higashida K, Taguchi S (2013) Modest hypoxia significantly reduces triglyceride content and lipid droplet size in 3T3-L1 adipocytes. Biochem Biophys Res Commun 440(1):43–49
Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM (2010) Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab 95(8):4052–4055
Dowman JK, Tomlinson JW, Newsome PN (2010) Pathogenesis of non-alcoholic fatty liver disease. QJM 103(2):71–83
Jouet P, Sabate JM, Maillard D, Msika S, Mechler C, Ledoux S, Harnois F, Coffin B (2007) Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg 17(4):478–485
Polotsky VY, Patil SP, Savransky V et al (2009) Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med 179(3):228–234
Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R (2013) Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev 14(5):417–431
Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY (2011) Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 19(11):2167–2174
Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY (2006) Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Physiol Genomics 25(3):450–457
Goda N, Kanai M (2012) Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol 95(5):457–463
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR α in energy metabolism and vascular homeostasis. J Clin Invest 116(3):571–580
Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH (2009) Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol 29(16):4527–4538
Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, Shah YM (2011) Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 54(2):472–483
Nishiyama Y, Goda N, Kanai M et al (2012) HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol 56(2):441–447
Tailleux A, Wouters K, Staels B (2012) Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta 1821(5):809–818
Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347(6294):645–650
Sahebkar A, Chew GT, Watts GF (2014) New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother 15(4):493–503
Hamaguchi T, Iizuka N, Tsunedomi R et al (2008) Glycolysis module activated by hypoxia-inducible factor 1α is related to the aggressive phenotype of hepatocellular carcinoma. Int J Oncol 33(4):725–731
Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, Mandrekar P, Szabo G (2011) Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology 53(5):1526–1537
Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, Boren J, Olofsson SO (2006) Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol 26(7):1566–1571
Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, Stahlman M, Olofsson SO, Hulten LM (2006) Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol 26(8):1871–1876
Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK (2014) Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie 96:121–129
Kohjima M, Enjoji M, Higuchi N et al (2007) Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 20(3):351–358
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115(5):1343–1351
Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ (2008) Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 48(6):1810–1820
Postic C, Magnuson MA (2000) DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26(2):149–150
Haase VH, Glickman JN, Socolovsky M, Jaenisch R (2001) Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc Natl Acad Sci USA 98(4):1583–1588
Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG Jr (2006) Failure to prolyl hydroxylate hypoxia-inducible factor α phenocopies VHL inactivation in vivo. EMBO J 25(19):4650–4662
Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY (2012) Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One 7(10):e46562
Ochiai D, Goda N, Hishiki T, Kanai M, Senoo-Matsuda N, Soga T, Johnson RS, Yoshimura Y, Suematsu M (2011) Disruption of HIF-1α in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun 415(3):445–449
Le Roy T, Llopis M, Lepage P et al (2013) Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 62(12):1787–1794
Diamant M, Blaak EE, de Vos WM (2011) Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev 12(4):272–281
Mehal WZ (2012) HIF-1α is a major and complex player in alcohol induced liver diseases. J Hepatol 56(2):311–312
Moon JO, Welch TP, Gonzalez FJ, Copple BL (2009) Reduced liver fibrosis in hypoxia-inducible factor-1α-deficient mice. Am J Physiol Gastrointest Liver Physiol 296(3):G582–G592
Wang XL, Suzuki R, Lee K et al (2009) Ablation of ARNT/HIF1β in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab 9(5):428–439
Ni HM, Bhakta A, Wang S, Li Z, Manley S, Huang H, Copple B, Ding WX (2014) Role of hypoxia inducing factor-1β in alcohol-induced autophagy, steatosis and liver injury in mice. PLoS One 9(12):e115849
Rahtu-Korpela L, Karsikas S, Horkko S et al (2014) HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63(10):3324–3333
Hyvarinen J, Hassinen IE, Sormunen R, Maki JM, Kivirikko KI, Koivunen P, Myllyharju J (2010) Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem 285(18):13646–13657
Wei K, Piecewicz SM, McGinnis LM et al (2013) A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat Med 19(10):1331–1337
Heindryckx F, Kuchnio A, Casteleyn C, Coulon S, Olievier K, Colle I, Geerts A, Libbrecht L, Carmeliet P, Van Vlierberghe H (2012) Effect of prolyl hydroxylase domain-2 haplodeficiency on the hepatocarcinogenesis in mice. J Hepatol 57(1):61–68
Coulon S, Legry V, Heindryckx F et al (2013) Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology 57(5):1793–1805
Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P (2010) From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler Thromb Vasc Biol 30(12):2331–2336
Acknowledgments
The authors would like to thank Margot Guillemin for her critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
S.L. and X.V. received a research Grant from the Fund for Scientific Research (FWO Flanders, FWO15/ASP/146 and 1700214N, respectively). H.V.V. is a senior clinical researcher of the FWO Flanders.
Conflict of interest
The authors declare no conflict of interest.
Additional information
L. Devisscher and A. Geerts contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Lefere, S., Van Steenkiste, C., Verhelst, X. et al. Hypoxia-regulated mechanisms in the pathogenesis of obesity and non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 73, 3419–3431 (2016). https://doi.org/10.1007/s00018-016-2222-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2222-1