Summary.
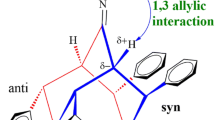
The stereoselectivities of the quaternization reactions of (4aα,8aβ,9aβ,10aα)- and (4aα,8aα,9aβ,10aα)-tetradecahydro-10-methylacridine with methyl- and ethyl iodide as well as those of (4aα,8aβ,9aβ,10aα)- and (4aα,8aα,9aβ,10aα)-10-ethyl-tetradecahydroacridine with methyl iodide were investigated using 13C NMR spectroscopy including 13C-labelling where appropriate. The methylations of both N-methyl amines occur by predominant (60% and 75%, respectively) equatorial approach, their ethylations occur sterospecifically by equatorial approach, and the methylations of the N-ethyl amines occur by highly stereoselective (> 90%) axial approach of the quaternizing reagent.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received October 27, 1999. Accepted November 22, 1999
Rights and permissions
About this article
Cite this article
Potmischil, F., Herzog, H., Buddrus, J. et al. Hydroacridines XXI [1]. 13C NMR Spectroscopic Investigation of the Stereoselectivities of Quaternizations of N-Alkyl Derivatives of (4aα,8aβ,9aβ,10aα)- and (4aα,8aα,9aβ,10aα)-Tetradecahydroacridine. Monatshefte für Chemie 131, 345–352 (2000). https://doi.org/10.1007/PL00010310
Issue Date:
DOI: https://doi.org/10.1007/PL00010310