Summary
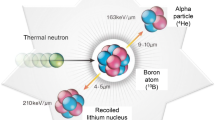
The boron neutron capture therapy is based on the reaction occuring between the isotope10B and thermal neutrons. A low energy neutron is captured by the nucleus and it disintegrates into two densly ionising particles, Li nucleus and He nucleus (α particle), with high biological effectiveness. On the basis of comprehensive preclinical investigations in the frame of the European Collaboration with Na2B12H11SH (BSH), as boron delivery agent, the first European phase I, clinical trial was designed at the only available epithermal beam in Europe, at the High Flux Reactor, Petten, in the Netherland. The goal of this study is to establish the safe BNCT dose for cranial tumors under defined conditions.
BNCT is applied as postoperative radiotherapy in 4 fractions, after removal of the tumor for a group of patients suffering from glioblastoma, who would have no benefit from conventional treatment, but have sufficient life expectancy to detect late radiation morbidity due to BNCT.
The starting dose is set at 80% of the dose where neurological effects occured in preclinical large animal experiments following a single fraction. The radiation dose will be escalated, by constant boron concentration in blood, in 4 steps for cohorts of ten patients, after an observation period of at least 6 months after the end of BNCT of the last patient of a cohort.
The adverse events on healthy tissues due to BSH and due to the radiotherapy will be analysed in order to establish the maximal tolerated dose and dose limiting toxicity. Besides of the primary aim of this study the survival will be recorded. The first patient was treated in October 1997, and further four patients have been irradiated todate. The protocol design proved to be well applicable, establishing the basis for scientific evaluation, for performance of safe patient treatment in a very complex situation and for opening the possibility to perform further clinical research work on BNCT.
Similar content being viewed by others
References
Ceberg CP, Brun A, Kahl SB, et al. A comparative study on the farmacokinetics and biodistribution of boronated porphyrin (BOPP) and sulphydril boron hydride (BSH) in thr RG2 rat glioma model. J Neurosurg 1995;83:86–92.
Ceberg CP, Persson A, Brun A, et al. Performance of BSH in patients with astrocytoma grades III–IV — a basis for boron neutron capture therapy. J Neurosurg 1995;83:79–85.
European Organization for Research and Treatment of Cancer BNCT Study Group. Protocol 11961 Postoperative Treatment of Glioblastoma with BNCT at the Petten Irradiation Facility Phase I Clinical Trial, 1996.
Fankhauser H, Stragliotto G, Zbinden P. Borocaptate sodium (BSH) pharmacokinetics in glioma patients. In: Gabel D, Moss RL (eds.). Boron Neutron Capture Therapy. Toward Clinical Trials of Glioma Treatment. New York, Plenum Press, 1992;155–62.
Gavin PR, Huiskamp R, Wheele, FJ, et al. Large animal normal tissue tolerance using an epithermal neutron beam and borocaptate sodium. Strahlenther Onkol 1993;169:48–5.
Haritz D, Gabel D, Huiskamp R. Clinical Phase-I Study of Na2B12H11SH (BSH) in Patients with Malignant Glioma as Precondition for Boron Neutron Capture Therapy (BNCT). Int J Radiat Oncol Biol Phys 1994; 28:1175–81.
Haritz D, Piscol K, Gabel D. The biodistribution of BSH in patients with malignant glioma. In: Allen BJ, Moore DE, Harrington BV (eds.). Progress in Neutron Capture Therapy for Cancer. New York, Plenum Press, 1992;557–60.
Haselsberger K, Radner H, Pendl G. Boron neutron capture therapy: Boron biodistribution and pharmacokinetics of Na2B12H11SH in patients with glioblastoma. Cancer Res 1994;54:6318–20.
Hatanaka H, Nakagawa Y. Clinical results of Long-surviving Brain Tumor Patients Who Underwent Boron Neutron Capture Therapy. Int J Radiat Oncol Biol Phys 1994;28:1061–6.
Mehta SC, Lu DR. Interspecies pharmacokinetic scaling of BSH in mice, rats, rabbits, and humans. Biopharm Drug Dispos 1995;16:735–44.
Moss RL, Casado J, Ravensberg K, et al. The completed BNCT facility at HFR Petten In: Abstracts of the Seventh International Symposium on Neutron Capture Therapy for Cancer. p: 135 Zürich, 4–7 September, 1996.
Nakagawa Y, Kyonghon P, Kitamura K, et al. What were important factors in patient’s treated by boron neutron capture therapy in Japan In: Abstracts of the Seventh International Symposium on Neutron Capture Therapy for Cancer. p: 164 Zürich, 4–7 September, 1996.
Otersen B, Haritz D, Grochulla F, et al. Binding and immunohistochemical localization of Na2B12H11SH to tumor tissue of glioma patients in boron neutron capture therapy. In: Mishima Y (ed.): Neutron Capture Therapy for Human Cancers. New York, Plenum Press, 1995, submitted.
Pavy J-J, Denekamp J, Letschert J, et al. EORTC late effects working group Late effects toxicity scoring: the SOMA scale.
Sauerwein W. The clinical project at HFR Petten — A status report. In: Abstracts of the Seventh International Symposium on Neutron Capture Therapy for Cancer. p: 166 Zürich, 4–7 September, 1996.
Stragliotto G, Frankhauser H. Biodistribution and pharmacokinetics of boron-sulfhydryl for boron neutron capture therapy in patients with intracranial tumors. Neurosurgery 1995;36:285–93.
Wheeler FJ. Radiation transport in tissue by Monte Carlo=Version X02. Idaho National Engineering Laboratory report no. EGG-BNCT-11178, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hideghéty, K., Sauerwein, W., Haselsberger, K. et al. Postoperative treatment of glioblastoma with BNCT at the Petten irradiation facility (EORTC Protocol 11961). Strahlenther Onkol 175 (Suppl 2), 111–114 (1999). https://doi.org/10.1007/BF03038907
Issue Date:
DOI: https://doi.org/10.1007/BF03038907