Abstract
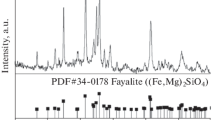
In this study, the interaction between magnetite and the additive olivine was studied after oxidation as well as after isothermal reduction at temperatures in the 500°–1,300° C range. In the olivine sample, the forsteritic olivine particles react partly during the oxidation pretreatment to form magnesioferrite and vitreous silica along the particle corona. This breakdown of the olivine particles during oxidation liberates magnesium from the particles, which do not react until temperatures of above 1,150° C in reducing atmosphere. When the hematite in the sample is reduced, and when the temperature is high enough to allow solid-state diffusion at ∼800° C, the magnesium of the magnesioferrite redistributes, so that the magnesium concentration approaches the same level throughout the iron oxide structure. For magnetite, this did not occur until 800° C. At 1,000° C, this magnesium reacts further with the silica in the glassy slag phase, which crystallizes into fayalitic olivine. At this temperature, the magnesium has diffused over distances of more than 600 µm from large olivine particles after 2 hrs reduction. From this point, the primary slag phase in the pellet, until melting, is solid fayalite. Upon reduction to metal, the metallization front concentrates the MgO in the remaining wustite, which can lead to MgO levels of up to 10 mole% locally. The melting point of the fayalite is raised from 1,145° C to a melting range of 1,238–1,264° C due to the MgO increase, as estimated based on phase diagrams, which were adapted to the pellets tested. Much of the olivine that remained unaltered in the oxidation process will be encapsulated by iron before the particles begin to dissolve in reducing conditions and, therefore, plays no role in the reduction before final melting of the particles occurs.
Similar content being viewed by others
References
Biswas, A.K., 1981, Principles of Ironmaking Blast Furnace, Cootha Publishing House, Australia, 74 pp.
Bradshaw, A.V., and Matyas, A.G.,1976, “Structural changes and kinetics in the gaseous reduction of hematite,” Metall. Mater. Trans. B, Vol. 7b, No. 1, pp. 81–87.
Edström, J.O., 1957 “Solid state diffusion in the reduction of hematite,” Jernkont. Ann., Vol. 141, pp. 809–836.
Forsmo, S.P.E., and Hägglund, A., 2003, “Influence of the olivine additive fineness on the oxidation of magnetite pellets,” Int J Mineral Process, Vol. 70, Nos.1–4, pp. 109–122.
Gränse, L., 1971, “The influence of slagforming additions on the swelling of pellets from very rich magnetite concentrates,” Proceedings of ICSTIS, Suppl. Trans. ISI Japan, Vol. 11, pp. 45–51.
Hallin, M., Thulin, D., and Tottie, M., 1994, “LKAB-Pellets in the Blast Furnace,” Proceedings of the 53rd Ironmaking Conference, March 20–23, 1994, Chicago, Vol. 53, AIME, pp. 287–291.
Hayes, P.C., and Grieveson, P., 1981, “Microstructural changes on the reduction of hematite to magnetite,” Metall. Trans. B, Vol. 12b, No. 2, pp. 579–587.
Lingtan, K., Yang, L., and Lu, W.-K., 1983, “The role of magnesia in iron ore pellets,” Scand. J. Metall, Vol. 12, No. 4, pp.166–176.
LKAB products, 2009, LKAB.
Niiniskorpi, V., 2004, Development of phases and structures during pelletizing of Kiruna magnetite ore, PhD thesis, Report 04-3, Åbo Akademi, Åbo, Finland.
Niiniskorpi, V., 2008, “Olivine reactions during pelletising of magnetite ore at high temperature under oxidising conditions,” 9th International Congress for Applied Mineralogy, Sept 8–10, 2008, Brisbane, Australia, Australasian Institute of Mining and Metallurgy, pp. 445–452.
Ryösä, E., 2008, Mineral reactions and slag formation during reduction of olivine blast furnace pellets, PhD thesis, Uppsala University, Uppsala, Sweden.
Semberg, P., Rutqvist, A., Andersson, C., and Björkman, B., 2011a, “Interactions between iron oxides and the additives quartzite, calcite and olivine in magnetite pellets,” ISIJ Int., Vol. 51, No. 2, pp. 173–180.
Semberg, P., Rutqvist, A., Andersson, C., and Björkman, B., 2011b, “Interaction between iron oxides and olivine in magnetite based pellets during reduction at temperatures below 1,000°C,” Ironmaking & Steelmaking, Vol. 38, No. 5, pp. 321–328.
Semberg, P., Andersson, C., and Björkman, B., 2013a, “Interaction between iron oxides and olivine in magnetite pellets during reduction to wustite at temperatures of 1,000–1,300° C,” ISIJ Int., Vol. 53, No. 3, pp. 391–398.
Semberg, P., Andersson, C., and Björkman, B., 2013b “Interaction between iron oxides and olivine in magnetite pellets during reduction to femet at temperatures of 1,000–1,300°C,” ISIJ Int., Vol, 53, No, 8, pp. 1341–1349.
Slagnes, S., and Sundqvist Okvist, L., 2008, “Olivine as slag conditioner in ironmaking, and its effect on processing characteristics and CO2 emission,” Annals — 3rd International Meeting on Ironmaking, Sept 22–26, 2008, Sao Luis City, Brazil, ABM, pp. 922–931.
Swann, P.R., and Tighe, N.J., 1977, “High voltage microscopy of the reduction of hematite to magnetite,” Metall. Trans. B, Vol. 8b, No. 2, pp. 479–487.
Thomalla, S., 1970, “Zum schwellverhalten von eisenerzpellets,” Stahl u. Eisen, Vol. 90, pp. 702–704.
Ünal, A., and Bradshaw, A.V., 1983, “Rate processes and structural changes in gaseous reduction of hematite particles to magnetite,” Metall. Trans B, Vol. 14b, No. 4, pp. 743–752.
Author information
Authors and Affiliations
Additional information
Paper number MMP-13-068.
Discussion of this peer-reviewed and approved paper is invited and must be submitted to SME Publications Dept. prior to November 30, 2014.
Rights and permissions
About this article
Cite this article
Semberg, P., Andersson, C. & Bjorkman, B. Interaction between iron oxides and olivine in magnetite pellets during reduction at 500°–1,300° C. Mining, Metallurgy & Exploration 31, 126–135 (2014). https://doi.org/10.1007/BF03402421
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402421