Abstract
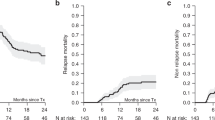
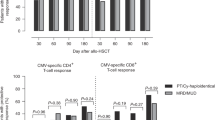
Improper T-cell reconstitution with its consequences, graft-vs-host disease (GvHD) and outbreak of viral infections, is the major cause of morbidity and mortality after hematopoietic stem cell transplantation (HSCT). To determine the factors affecting reconstitution of naive T-cells after non-myeloablative HSCT (NM-HSCT), the T-cell receptor excision circle (TREC) content was measured on a weekly basis in 24 transplanted patients with various malignant diseases. We analysed correlations of the results with the development of GvHD. In addition, in 11 chronic myeloid leukaemia (CML) patients, we correlated TREC and BCR-ABL transcript numbers. After HSCT, in most patients (22/24) TRECs became undetectable. In 12 patients, TRECs reappeared 3–4 months after HSCT, in 1 patient TRECs reappeared 5 months after HSCT, and in 11 patients TRECs remained negative for more than a year. All 11 patients who remained TREC-negative, developed acute GvHD grade 2–3, while only 6 out of 13 patients who recovered TRECs developed GvHD. We show that after non-myeloablative HSCT, thymopoiesis takes place and is affected by GvHD. Our results indicate that no recovery of TRECs after NM-HSCT (which most likely reflect the expansion of host-reactive co-transplanted mature T-cells) correlates with the onset of GvHD.
Similar content being viewed by others
References
Bahceci E, Epperson D, Douek DC, Melenhorst JJ, Childs RC, Barrett AJ, 2003. Early reconstitution of the T-cell repertoire after non-myeloablative peripheral blood stem cell transplantation is from post-thymic T-cell expansion and is unaffected by graft-versus-host disease or mixed chimaerism. Br J Haematol 122: 934–943.
Breit TM, Wolvers-Tettero IL, Bogers AJ, de Krijger RR, Wladimiroff JW, van Dongen JJ, 1994. Rearrangements of the human TCRD-deleting elements. Immunogenetics 40: 70–75.
de Villartay JP, Hockett RD, Coran D, Korsmeyer SJ, Cohen DI, 1988. Deletion of the human T-cell receptor delta-gene by a site-specific recombination. Nature 335: 170–174.
Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396: 690–695.
Fallen PR, McGreavey L, Madrigal JA, Potter M, Ethell M, Prentice HG, et al. 2003. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant 32: 1001–1014.
Hochberg EP, Chillemi AC, Wu CJ, Neuberg D, Canning C, Hartman K, et al. 2001. Quantitation of T-cell neogenesis in vivo after allogeneic bone marrow transplantation in adults. Blood 98: 1116–1121.
Kalwak K, Gorczynska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, et al. 2002. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol 118: 74–89.
Kong F, Chen CH, Cooper MD, 1998. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity 8: 97–104.
Kong FK, Chen CL, Six A, Hockett RD, Cooper MD, 1999. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci USA 96: 1536–1540.
Kreuzer KA, Schmidt CA, Schetelig J, Held TK, Thiede C, Ehninger G, et al. 2002. Kinetics of stem cell engraftment and clearance of leukaemia cells after allogeneic stem cell transplantation with reduced intensity conditioning in chronic myeloid leukaemia. Eur J Haematol 69: 7–10.
Michalek J, Collins RH, Durrani HP, Vaclavkova P, Ruff LE, Douek DC, et al. 2003a. Definitive separation of graft-versus-leukemia- and graft-versus-host-specific CD4+ T cells by virtue of their receptor beta loci sequences. Proc Natl Acad Sci USA 100: 1180–1184.
Michalek J, Collins RH, Hill BJ, Brenchley JM, Douek DC, 2003b. Identification and monitoring of graft-versus-host specific T-cell clone in stem cell transplantation. Lancet 361: 1183–1185.
Savage WJ, Bleesing JJ, Douek D, Brown MR, Linton GM, Malech HL, et al. 2001. Lymphocyte reconstitution following non-myeloablative hematopoietic stem cell transplantation follows two patterns depending on age and donor/recipient chimerism. Bone Marrow Transplant 28: 463–471.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. 1998. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91: 756–763.
Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, et al. 1999. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood 93: 467–480.
Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG, 2002. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation 73: 1154–1158.
Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C, et al. 1999. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant 23: 1055–1060.
Verschuren MC, Wolvers-Tettero IL, Breit TM, Noordzij J, van Wering ER, van Dongen JJ, 1997. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J Immunol 158: 1208–1216.
Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. 2001. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood 97: 1458–1466.
Zhang L, Lewin SR, Markowitz M, Lin HH, Skulsky E, Karanicolas R, et al. 1999. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med 190: 725–732.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Przybylski, G.K., Kreuzer, KA., Siegert, W. et al. No recovery of T-cell receptor excision circles (TRECs) after non-myeloablative allogeneic hematopoietic stem cell transplantation is correlated with the onset of GvHD. J Appl Genet 48, 397–404 (2007). https://doi.org/10.1007/BF03195239
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03195239