Summary
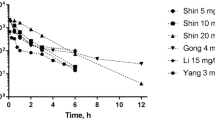
From this study it is proposed that the clearance of anti-cancer drugs may be best estimated if they follow the following trend: (i) that if the exponent of the allometric equation (clearance vs body weight) lies between 0.55–0.75 (0.75 was chosen after Kleiber’s power rule (18)), then a simple allometric equation can predict the clearance reasonably well; (ii) if the exponent of the allometric equation lies between 0.75 to approximately 0.90, clearance can be reasonably predicted using CL × MLP; and (iii) if the exponent of the allometric equation is ≥0.90, the clearance can be predicted using the product of clearance and brain weight.
Similar content being viewed by others
References
Heusner A.A. (1987): What does the power function reveal about structure and function in animals of different size. Annu. Rev. Physiol., 78, 121–133.
McNamara P.J. (1991): Interspecies scaling in pharmacokinetics. In: Welling P.G., Tse F.L.S., Dighe S.V. (Eds) Pharmaceutical Bioequivalence. New York. Marcel Dekker, pp. 267–300.
Boxenbaum H., Murray W., Markin, R., Ciraulo D. (1985): In: Barnett G., Nora C.C. (Eds) Pharmacokinetics and Pharmacodynamics of Psychoactive Drugs. Forest City, CA, USA. Biomedical Publications, p. 93.
Voisin E.M., Ruthsatz M., Collins J.M., Hoyle P.C. (1990): Extrapolation of animal toxicity to humans: interspecies comparisons in drug development. Reg. Toxicol. Pharmacol., 12, 107–116.
Chappel W.R., Mordenti J. (1991): Extrapolation of toxicological and pharmacological data from animals to humans. Adv. Drug. Res., 20, 1–116.
Boxenbaum H. (1984): Interspecies pharmacokinetic scaling and the evolutionary comparative paradigm. Drug Metab. Rev., 15, 1071–1121.
Dedrick R.L., Forrester D.D., Cannon J.N., Dareer S.M.E., Mellett B.L. (1973): Pharmacokinetics of 1-β-d-arabinofuranosylcytosine (Ara-C) deamination in several species. Biochem. Pharmacol., 22, 2405–2417.
King F.G., Dedrick R.L., Farris F.F. (1986): Physiological pharmacokinetic modeling ofcis-dichlorodiamineplatinum (II) (DDP) in several species. J. Pharmacokinet. Biopharm., 14, 131–155.
Paxton J.W., Kim S.N., Whitfield L.R. (1990): Pharmacokinetic and toxicity of the antitumor agents amascrine and CI-921, a new analogue, in mice, rats, rabbits, dogs and humans. Cancer Res., 50, 2692–2697.
McGovern P.J., Williams M.G., Stewart J.C. (1988): Interspecies comparison of acivicin pharmacokinetics. Drug. Metab. Dispos., 16, 18–22.
Kaye S.B., Workman P., Graham P.A., Cassidy J., Jorell D. (1993): Pharmacokinetics and early clinical studies of selected new drugs. In: Workman P., Graham M.A. (Eds) Pharmacokinetics and Cancer Chemotherapy. Cold Spring Harbor, Cold Spring Harbor Laboratory Press, pp 371–396.
Mehta S.C., Lu D.R. (1995): Interspecies pharmacokinetic scaling of BSH in mice, rats, rabbits, and humans: Biopharm. Drug. Dispos., 16, 735–744.
Sacher G. (1959): Relation of lifespan to brain weight and body weight in mammals. In: Wolstenholme G.E.W., O’Connor M. (Eds) CIBA Foundation Colloquium on Aging. London, Churchill, pp. 115–133.
Dedrick R.L., Bischoff K.B., Zaharko D.Z. (1970): Interspecies correlation of plasma concentration history of methotrexate (NSC-740). Cancer Chemother. Rep., Part 1, 54, 95–101.
Mordenti J. (1986): Man versus beast. J. Pharm. Sci., 75, 1028–1040.
Boxenbaum H. (1982): Interspecies scaling, allometry, physiological time and the ground plan of pharmacokinetics. J. Pharmacokinet. Biopharm., 10, 201–227.
Dedrick R.L. (1973): Animal scale-up. J. Pharmacokinet. Biopharm., 1, 435–461.
Kleiber M. (1961): In: The Fire of Life, an Introduction to Animal Energetics. New York, Wiley.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mahmood, I. Interspecies scaling: predicting clearance of anticancer drugs in humans. European Journal of Drug Metabolism and Pharmacokinetics 21, 275–278 (1996). https://doi.org/10.1007/BF03189726
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189726