Abstract
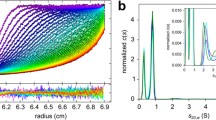
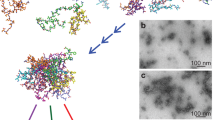
Pulsed field gradient nuclear magnetic resonance technique was applied to measure the self-diffusion coefficient of Aβ1–40 peptide in trifluoroethanol (TFE) and mixed solvent TFE-water (D2O) buffer (pD 7.8) at 293 K. The data were analyzed on the basis of the Stokes model and the hardsphere approach was used to estimate self-diffusion coefficients. It was found that the extent of the Aβ1–40 aggregation in TFE solutions depends on the concentration of the peptide and the sample preparation protocol. After soft mixing, i.e., without any additional mechanical pretreatment of the peptide, the peptide is present in the monomeric form in TFE solutions. However, the additional water-bath sonication of the sample during the dissolution of Aβ1–40 in TFE enforces oligomerization of the peptide with the size of aggregates ranging from tetra- to hexamers. An increase of D2O in the mixed TFE-D2O solvent of up to 75% leads to the aggregation of the larger part of the peptide. However, the components of self-diffusion coefficients related to low-mass Aβ1–40 oligomers (dimers and trimers) were not observed in the diffusion decay curves. The most probable explanation is that dimers and trimers are not the principal intermediate species in the aggregation of Aβ1–40 peptide.
Similar content being viewed by others
References
Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L., Beyreuther K.: Proc. Natl. Acad. Sci. USA82, 4245–4249 (1985)
Haass C., Selkoe D.J.: Cell75, 1039–1042 (1993)
Iversen L.L., Mortishire-Smith R.J., Pollack S.J., Shearman M.S.: Biochem. J.311, 1–16 (1995)
Rochet J.-C., Lansbury Jr. P.T.: Curr. Opin. Struct. Biol.10, 60–68 (2000)
Kosik K.S.: Proc. Natl. Acad. Sci. USA96, 2574–2576 (1999)
Bucciantini M., Giannoni E., Chiti F., Baroni F., Formigli L., Zurdo J., Taddei N., Ramponi G., Dobson C.M., Stefani M.: Nature416, 507–511 (2002)
Lansbury P.T. Jr.: Proc. Natl. Acad. Sci. USA96, 3342–3344 (1999)
Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C., Cotman C.W., Glabe C.: Science300, 486–489 (2003)
Antzutkin O.N., Balbach J.J., Leapman R.D., Rizzo N.W., Reed J., Tycko R.: Proc. Natl. Acad. Sci USA97, 13045–13050 (2000)
Lorenzo A., Yuan M., Zhang Z., Paganetti P.A., Sturchler-Pierrat C., Staufenbiel M., Mautino J., Vigo F.S., Sommer B., Yankner B.A.: Nat. Neurosci.3, 460–464 (2000)
Jarvet J., Damberg P., Bodell K., Eriksson L.E.G., Gräslund A.: J. Am. Chem. Soc.122, 4261–4268 (2000)
Danielsson J., Jarvet J., Damberg P., Gräslund A.: Magn. Reson. Chem.40, S89-S97 (2002)
Price W.S., Tsuchiya F., Arata Y.: J. Am. Chem. Soc.121, 11503–11512 (1999)
Jones J.A., Wilkins D.K., Smith L.J., Dobson C.M.: J. Biomol. NMR10, 199–203 (1997)
Wilkins D.K., Grimshaw S.B., Receveur V., Dobson C.M., Jones J.A., Smith L.J.: Biochemistry38, 16424–16431 (1999)
Yao S., Howlett G.H., Norton R.S.: J. Biomol. NMR16, 109–119 (2000)
Tseng B.P., Ester W.P., Clish C.B., Stimson E.R., Ghilardi J.R., Vinters H.V., Mantyh P.W., Lee J.P., Maggio J.E.: Biochemistry38, 1424–10431 (1999)
Hou L., Shao H., Zhang Y., Li H., Menon N.K., Neuhaus E., Brewer J.M., Byeon I.-J.L., Ray D.G., Vitek M.P., Iwashita T., Makula R.A., Przybyla A.B., Zagorski M.: J. Am. Chem. Soc.126, 1992–2005 (2005)
Narayanan S., Reif B.: Biochemistry44, 1444–1452 (2005)
Krishnan V.V.: J. Magn. Reson.124, 468–473 (1997)
Stejskal E.O., Tanner J.E.: J. Chem. Phys.42, 288 (1965)
Shuck P., MacPhee C.E., Howlett G.J.: Biophys. J.74, 466–474 (1998)
Fushman D. Varadan R., Assfalg M., Walker O.: Prog. Nucl Magn. Reson. Spectrosc.44, 189–214 (2004)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Filippov, A., Sulejmanova, A., Antzutkin, O. et al. Diffusion and aggregation of Alzheimer’s Aβ1–40 peptide in aqueous trifluoroethanol solutions as studied by pulsed field gradient NMR. Appl. Magn. Reson. 29, 439 (2005). https://doi.org/10.1007/BF03167174
Received:
Revised:
DOI: https://doi.org/10.1007/BF03167174