Abstract
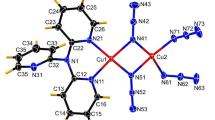
The [Cd(N(CH3)3)2Ni(CN)4] complex crystallizes in a tetragonal system, space group 14/mmm with two formula units per unit cell (XRD, Rigaku AFC-6A diffractometer, λ MoKα, ω/2θ scan mode, θmax = 38ℴ, 635 observed unique reflections, 53 parameters, R = 0.027). The structure consists of parallel polymer layers made up of coordinated metal atoms and bridging cyanides. The octahedral environment of Cd(II) involves six nitrogen atoms of the four cyanide groups in the layer plane (2.323(4) Å) and the two trimethylamine ligands in the transposition (2.42(1) Å). The square-planar environment of Ni(II) consists of four carbon atoms of the cyanide ligands (1.857(3) Å). The layers are packed according to van der Waals type; the “hollows” near the nickel atoms are filled by the “hills” of the trimethylamino groups from the neighboring layer (the interlayer distance is 7 Å). The spatial complementarity of the layers leads to close packing of the complex and explains the lack of a clathrate-forming ability in the latter. The trimethylamine ligands here play the same role as guest molecules in Hofmann clathrates, stabilizing the planar polymer structure of the complex. This phenomenon is called contact self-stabilization.
Similar content being viewed by others
References
T. Iwamoto, in:Inclusion Compounds, Vol. I, Academic Press, London (1984), pp. 29–57.
K. A. Hoffmann and F. Z. Kuspert,Z. Anorg. Chem.,15, 204–207 (1897).
H. M. Powell and J. H. Rayner,Nature,163, 566–567 (1949).
H. M. Powell,J. Chem. Soc, 61–73 (1948).
T. Iwamoto,J. Incl. Phenom.,24, 61–132 (1996).
N. V. Gerbeleu, Yu. A. Simonov, V. B. Arion, et al.,Inorg. Chem.,31, 3264–3268 (1992).
Yu. A. Simonov, M. S. Fonari, J. Lipkowski, et al.,J. Incl. Phenom.,24, 149–161 (1996).
G. V. Gavrilova, N. V. Kislykh, and V. A. Logvinenko,J. Therm. Anal.,33, 229–235 (1988).
E. Jona and R. Boca,J. Incl. Phenom.,14, 65–71 (1992).
P. Timmerman, R. H. Vreekamp, R. Hulst, et al.,Chem. Eur. J.,3, 1823–1832 (1997).
D. V. Soldatov, J. A. Ripmeester, S. I. Shergina, et al.,J. Am. Chem. Soc,121, 4179–4188 (1999).
D. V. Soldatov, B. A. Kolesov, J. Lipkowski, and Yu. A. Dyadin,Zh. Strukt. Khim.,38, 976–987 (1997).
Yu. A. Dyadin and N. V. Kislykh,Mendeleev Commun., 134–136 (1991).
D. V. Soldatov, V. A. Logvinenko, and Yu. A. Dyadin,Zh. Neorg. Khim.,40, 324–328 (1995).
Yu. A. Dyadin, D. V. Soldatov, V. A. Logvinenko, and J. Lipkowski,J. Coord. Chem.,37, 63–75 (1996).
D. V. Soldatov, Yu. A. Dyadin, E. A. Ukraintseva, et al.,J. Incl. Phenom.,26, 269–280 (1996).
E. A. Ukraintseva, D. V. Soldatov, V. A. Logvinenko, and Yu. A. Dyadin,Mendeleev Commun., 102–104 (1997).
Y. Sasaki,Bull. Chem. Soc. Jpn.,42, 2412 (1969).
S. Nishikiori, T. Kitazawa, R. Kuroda, and T. Iwamoto,J. Incl. Phenom.,7, 369–377 (1989).
S. Nishikiori and T. Iwamoto,Chem. Lett., 1035–1038 (1982).
S. Nishikiori and T. Iwamoto,ibid., 1129–1130 (1983).
S. Nishikiori and T. Iwamoto,ibid., 319–322 (1984).
S. Nishikiori and T. Iwamoto,Anal. Sci.,4, 25–30 (1988).
S. Nishikiori and T. Iwamoto,Zh. Strukt. Khim.,40, No. 5, 898–926 (1999).
G. M. Sheldrick,Acta Crystal.,A46, 467–473 (1990).
G. M. Sheldrick,ibid.,A49 (Suppl.), C53 (1993).
A. I. Kitaigorodskii,Molecular Crystals [in Russian], Nauka, Moscow (1971).
Yu. V. Zefirov and P. M. Zorkii,Usp. Khim.,64, 417–524 (1995).
A. Bondi,J. Phys. Chem.,68, 441–451 (1964).
J. Ohyama, R. Tsuchiya, A. Uehara, and E. Kyuno,Bull. Chem. Soc. Jpn.,50, 410–414 (1977).
S. Nishikiori, T. Iwamoto, and Y. Yoshino,ibid.,53, 2236–2240 (1980).
E. A. Ukraintseva, D. V. Soldatov, Yu. A. Dyadin, et al.,Mendeleev Commun., 123–125 (1999).
Author information
Authors and Affiliations
Additional information
Translated fromZhurnal Strukturnoi Khimii, Vol. 40, No. 5, pp. 927–934, September–October, 1999.
Rights and permissions
About this article
Cite this article
Iwamoto, T., Nishikiori, S., Dyadin, Y.A. et al. Structure of the catena-bis(trimethylamine)cadmium(II)-tetra-μ-cyanonickelate(II) complex and contact self-stabilization of molecules. J Struct Chem 40, 750–756 (1999). https://doi.org/10.1007/BF02903450
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903450