Summary
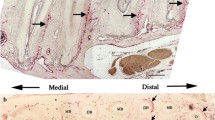
Tenascin is a major glycoprotein constituent of the extracellular matrix with a strong affinity to fibronectin; its distribution is believed to be temporarily and spatially limited. Tenascin gene expression is increased during wound healing processes. As repair mechanisms in chronic liver diseases resemble wound healing we studied tenascin gene expression in rat liver and in isolated rat liver cells. In normal rat liver a tenascin specific antiserum stains sinusoidal cells with fiber-like prolongations, which at the same time are desmin-positive (ITO-cells). In the CCl4-acutely-damaged liver a strong tenascin staining is detected in cells located among the mononuclear cells of the inflammatory infiltrates in the areas of necrosis and in cells of the sinusoids. In CG4-chronically-damaged liver a strong tenascin staining is demonstrable in the connective tissue septa. In both cases, many of the tenascin-positive cells can be identified as desmin-positive by means of the double-staining fluorescence technique. The wall of larger vessels is always tensacin-negative. The staining pattern obtained with a fibronectin-specific antiserum is somewhat comparable with that of tenascin but the vessel wall was positive. Hepatocytes, Kupffer cells, ITO-cells and endothelial cells were isolated from rat liver and studied for their capacity to express the tenascin gene. Biosynthetically labeled tenascin was immunoprecipated from supernatants and cell lysates obtained from cultured ITO-cells and to a much lesser extent from intracellular lysates obtained from endothelial cells; its synthesis in ITO-cells increased during the time in culture. Tenascin was also identified immuno-cytochemically in increasing amount in ITO-cells in culture. We conclude that ITO-cells may play a major role in tenascin synthesis during liver fibrogenesis.
Similar content being viewed by others
References
Bourdon MA, Wikstrand CJ, Furthmayer H, Mathews TH, Bigner DD (1983) Human glioma mesenchymal extracellular matrix antigen defined by monoclonal antibodies. Cancer Res43:2796–2805
Chiquet M, Fambrough DM (1984) Chick myotendinous antigen I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol 98:1926–1936
Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T (1986) Tenascin: An extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 47:131–139
Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M (1988) Tenascin interferes with fibronectin action. Cell 53:383–390
Chiquet-Ehrismann R, Kalla P, Pearson CA (1989) Participation of tenascin and transforming growth factor β in reciprocal epithelial mesenchymal interactions of MCF 7 cells and fibroblasts. Cancer Res 49:4322–4325
Cossell L (1966) Über das Auftreten von Basalmembranen an den Lebersinusoiden. Beiträge zur Pathologie 134:103–122
Davis BH (1988) Transforming growth factor β responsiveness is modulated by the extracellular collagen matrix during hepatic Ito cell culture. J Cell Physiol 136:547–553
Dijkstra CD, Döpp EA, Joling P, Kraal G (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulation in the rat recognized by monoclonal antibodies EDI, ED2 and ED3. Immunology 54:589–599
Grumet M, Hoffman S, Crossin KL, Edelman GM (1985) Cytoactin an extracellular matrix protein of neural and non neural tissues that mediates glia-neuron-interaction. Proc Natal Acad Sci USA 82:8075–8079
Gulcher JR, Nies DE, Marton LS, Steffansson K (1989) An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci USA, 86:1588–1592
Inaguma Y, Kusakabe M, Mackie EJ, Pearson AC, Chiquet-Ehrismann R, Sakaura T (1988) Epithelial induction of stromal tenascin in the mouse mammary gland: From embryogenesis to carcinogenesis. Dev Biol 128:245–255
Jones FS, Hoffman S, Cunningham BA, Edelman GM (1989) A detailed structural model of cytoactin: Protein homologies alternative RNA splicing and binding regions. Proc Natl Acad Sci USA, 86:1905–1909
Knook DL, Blansjaar N, Sleyster E Ch (1977) Isolation and characterisation of Kupffer and endothelial cells from the rat liver. Exp Cell Res 109:317–329
Kruse Y, Keilhauer G, Faissner A, Timpl R, Schachner M (1985) The J1 glycoprotein a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature 316:146–148
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680
De Leeuw AM, Bareids R, Zanger R De, Knook DL (1982) Primary cultures of endothelial cells of the rat liver. Cell Tiss Res 223:201–215
De Leeuw AM, MacCarthy SP, Geerts A, Knook DL (1984) Purified rat fat storing cells in culture divide and contain collagen. Hepatology 6:392–403
Lightner VA, Gumkowski F, Bigner DD, Erickson HP (1989) Tenascin hexabrachion in human skin: Biochemical identification and localization by light and electron microscopy. J Cell Biol 108:2483–2493
Mackie EJ, Thesleff I, Chiquet-Erishmann R (1989) Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol 108:1873–1890
Makie EJ, Halfter D, Liverani D (1988) Induction of tenascin in healing wound. J Cell Biol 107:2757–2767
Martinez-Hernandez A (1985) The heaptic extracellular matrix II.Electron immunohistochemical studies in rats with CCL4 induced cirrhosis. Lab Invest 53:166–186
Matsuoka M, Pham NT, Tsukamoto H (1989) Differential effects of interleukin-lα, tumor necrosis factor a and transforming growth factor β1 on cell proliferation and collagen formation by cultured fat storing cells. Liver 9:71–78
Matsuoka M, Tsukamoto H (1990) Stimulation of hepatic lipocyte collagen producted by Kupffer cell-derived transforming growth factor β: Implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology 11:599–605
Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden ER, Thorgisson SS (1990a) Cellular distribution of transforming growth factor β1 and procollagen types I, III and IV transcripts in carbon tetrachloride induced rat liver fibrosis. J Clin Invest 85:1833–1843
Nakatsukasa H, Evarts RP, Hsia CC, Thorgirsson SS (1990 b) Transforming growth factor β1 and type I procollagen transcripts during regeneration and early fibrosis of rat liver. Lab Invest 63:171–180
Oakes B, Batty AL, Handley CJ, Sanberg LB (1982) The synthesis of elastin, collagen and glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur J Cell Biol 27:34–36
Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R (1988) Tenascin cDNA cloning and induction by TGF-β. Embo J 7:2677–2981
Proctor E, Chatamra K (1982) High yield micronodular cirrhosis in the rat. Gastroenterology 83:1183–1190
Ramadori G, Lenzi M, Dienes HP, Meyer zum Büschenfelde KH(1983) Binding properties of mechanically and enzymatically isolated hepatocytes for IgG and C3. Liver 3:358–368
Ramadori G, Sipe JD, Dinarello CA, Mizel SB, Colten HR (1985) Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med 162:930–942
Ramadori G, Dienes HP, Burger R, Meuer S, Rieder H, Meyer zum Büschenfelde KH (1986) Expression of Ia antigens on guinea pig Kupffer cells. Studies with monoclonal antibodies. J Hepatol 2:208–217
Ramadori G, Rieder H, Knittel Th, Dienes HP, Meyer zum Büschenfelde KH (1987) Fat storing cells (FSC) of rat liver synthesize and secrete fibronectin. Comparison with hepatocytes. J Hepatol 4:190–197
Ramadori G, Rieder H, Theiss F, Meyer zum Büschenfelde KH (1989) Fat storing (ITO) cells of rat liver synthesize and secrete apolipoproteins: Comparison with hepatocytes. Gastroeneterology 97:163–172
Ramadori G, Schwögler S, Veith Th, Chung AE, Rieder H, Meyer zum Büschenfelde KH (1990) Fat storing (ITO)-cells (FSC) of the rat liver synthesize and secrete entactin (nidogen). Comparison with other hepatic and non-hepatic cells. Gastroenterology 98:A623
Ramadori G, Veit Th, Schwögler S, Dienes HP, Knittel Th, Rieder H, Meyer zum Büschenfelde KH (1990) Expression of the gene of the α-smooth-muscle-actin isoform in rat liver and in rat fat storing (ITO)-cells. Virchows Archiv B Cell Pathol 59:349–357
Schuppan D (1990) Structure of the extracellular matrix in normal and fibrotic liver: Collagens and glycoproteins. Sem Liv Dis 10:1–10
Spring J, Beck K, Chiquet-Ehrismann R (1989) Two contrary functions of tenascin: Dissection of the active sites by recombinant tenascin fragments. Cell 59:325–334
Seglen PD (1972) Preparation of rat liver cells. I effect of Ca2 + on enzymatic dispersion of isolated perfused liver. Exp Cell Res 74:450–454
Shaffner F, Popper H (1966) Electron microscopy of cirrhotic nodule. Tubularisation of the parenchyma by biliary hepatocytes. Lab Invest 15:801–817
Van Eyken P, Sciot R, Desmet VJ (1990) Expression of the novel extracellular matrix component tenascin in normal and diseased human liver. An immunohistochemical study. J Hepatol 11:45–52
Yokoi Y, Namishia T, Matsuzaki K, Miyazaki A, Yamguchi Y (1988) Distribution of ITO-cells in experimental hepatic fibrosis. Liver 8:42–52
Weiner FR, Giambrone MA, Czaja MJ, Shah A, Annoni G, Takahashi S, Eghbali M, Zern MA (1989) ITO-cell gene expression and collagen regulation. Hepatology 11:111–117
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. W. Gerok at the occasion of his 65th anniversary
Rights and permissions
About this article
Cite this article
Ramadori, G., Schwogler, S., Veit, T. et al. Tenascin gene expression in rat liver and in rat liver cells In vivo and in vitro studies. Virchows Archiv B Cell Pathol 60, 145–153 (1991). https://doi.org/10.1007/BF02899540
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02899540