Abstract
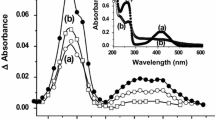
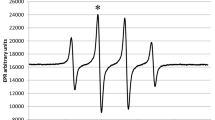
The mechanisms of reactions between CC13OO• radical and quercetin, rutin and epigallocatechin gallate (EGCG) have been studied using pulse radiolytic technique. It is suggested that the electron transfer reaction is the main reaction between CC13OO• radical and rutin, EGCG, but there are two main pathways for the reaction of CC13OO• radical with quercetin, one is the electron transfer reaction, the other is addition reaction. The reaction rate constants were determined. It is proved that quercetin and rutin are better CC13OO• radical scavengers than EGCG.
Similar content being viewed by others
References
Connor, H. D., Thurman, R. G., Galizi, M. D. et al., The formation of a novel free radical metabolite from carbon tetrachloridein the perfused rat liver andin vivo, J. Biol. Chem., 1986, 261(10): 4542–4548.
Moenig, J., Goebl, M., Asmus, K. D., Free radical one-electron versus hydroxyl radical-induced oxidation, Reaction of trichloromethylperoxyl radicals with simple and substituted aliphatic sulphides in aqueous solution, J. Chem. Soc, Perkin Trans. ll, 1985: 647–651.
Jovanovic, S. V., Simic, M. G., Redox properties of oxy and antioxidant radicals, in Oxygen Radicals in Biology and medicine (eds. Simic, M. G., Taylor, K. A., vonSonntag, C), New York: Plenum Press, 1988, 115–122
Neta, P., Huie, R. E., Maruthamuthu, P. et al., Solvent effects in the reactions of peroxyl radicals with organic reductants, Evidence for proton-transfer-mediated electron transfer, J. Phys. Chem., 1989, 93: 7654–7659.
Alfassi, Z. B., Huie, R. E., Kumar, M. et al., Temperature dependence of the rate constants for oxidation of organic compounds by peroxyl radicals in aqueous alcohol solutions, J. Phys. Chem., 1992, 96: 767–770.
Shen, X. H., Lind, J., Eriksen, T. E. et al., The reaction of the CC13OO• radical with indoles, J. Chem. Soc, Perkin Trans. 11, 1989: 555–562.
Yao, S. D., Sheng, S. G., Cai, J. H. et al., Nanosecond pulse radiolysis studies in China, Radiat. Phys. Chem., 1995, 46: 105–109.
Brault, D., Neta, P., Reactions of iron (III) porphyrins with peroxyl radicals derived from halothane and halomethanes, J. Phys. Chem., 1984, 88: 2857–2866.
Shen, X. H., Lind, J., Eriksen, T. E., Reactivity of the CC13OO• radical, Evidence for a first-order transformation, J. Phys. Chem., 1989, 93: 553–557.
Bors, W., Saran, M. Radical scavenging by flavonoid antioxidants, Free Radical Res. Comm., 1987, 2(4-6): 289–294.
Jovanovic, S. V., Steeken, S., Tosic, M. et al., Flavonoids as antioxidants, J. Am. Chem. Soc, 1994, 116: 4846–4851.
Jovanovic, S. V., Hara, Y., Steeken, S. et al., Antioxidant potential of gallocatechins, Apulse radiolysis and laser photolysis study, J. Am. Chem. Soc, 1995, 117: 9881–9888.
Rice-Evans, C., Miller, N. J., Paganga, G. et al., Structure-antioxidant activity relationships of flavonoids and phenolic acids, Free Radical Biol. Med., 1996, 20(7): 933–956.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, J., Wang, W., Pan, J. et al. Pulse radiolysis study on the mechanisms of reactions of CCl3OO. radical with quercetin, rutin and epigallocatechin gallate. Sc. China Ser. B-Chem. 44, 353–359 (2001). https://doi.org/10.1007/BF02879809
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02879809