Abstract
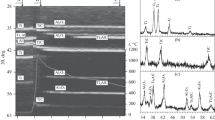
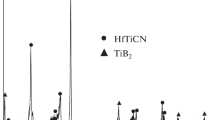
The reaction process of combustion synthesis for TiB2−Cu was investigated in detail using combustion-wave arresting experiment, X-ray diffraction (XRD) analysis, SEM analysis and differential thermal analysis (DTA). The XRD analysis results for the different parts of the quenched specimen show that TiCux intermetallic phase firstly forms with the propagation of combustion wave, and then Ti1.87B50 and Ti3B4 metastable phases come forth due to the diffusion of B atoms and finally the stable TiB2 phase forms because of the continuous diffusion of B atoms. The formation of TiB2 phase is not completed by one step, but undergoes several transient processes. The process of reaction synthesis for Ti−B−Cu ternary system can be divided into three main stages: melting of Cu and Ti, and the formation of Cu−Ti melt and few TiCux, TiBx intermetallic phases; large numbers of TiCux intermetallic phases formation and some fine TiB2 particles precipitation; and the TiB2 particles coarsening and the stable TiB2 and Cu two phases formation in the final product.
Similar content being viewed by others
References
A G Merzhanov. Combustion Processes that Synthesize Materials [J].Journal of Materials Processing Technology, 1996, 56(1–4): 222
Zou Zhengguang, Fu Zhengyi, Yuan Runzhang and Mai Liqiang. Nonlinear Dynamic Characteristics of Combustion Wave in SHS Process[J].Journal of Wuhan University of Technology-Mater. Sci. Ed., 2002, 17(1):23
A G Merzhanov, A S Rogachev, A S Mukasyan, B M Khusid. Macrokinetics of Structural Transformation During the Gasless Combustion of a Titanium and Carbon Powder Mixture[J].Combustion, Explosion and Shock Waves, 1990, 26(1):92
J C LaSalvia, D K Kim, R A Lipsett, M A Meryers. Combustion Synthesis in the Ti−C−Ni−Mo System[J].Metallurgical and Materials Transactions A, 1995, 26A(11): 3001
J Wong, E M Larson, J B Holt, P A Waide, B Rupp, R Frahm. Time-Resolved X-Ray Diffraction Study of Solid Combustion Reactions[J].Science, 1990, 249:1406
S D Dunmead, D W Readey, C E Semler. Kinetics of Combustion Synthesis in the Ti−C and Ti−C−Ni Systems[J].Journal of the American Ceramic Society, 1989, 71(12):2318
E L Zhang, S Y Zeng, B Yang, Qingchun Li, Mingzhen Ma. A Study on the Kinetic Process of Reaction Synthesis of TiC[J].Metallurgical and Materials Transactions A, 1999, 30A(4): 1147
J L Murray. The Cu−Ti (Copper-Titanium) System[J].Bulletin of Alloy Phase Diagrams, 1983, 4(1): 81
D J Chakrabarti, D E Laughlin. The B−Cu (Boron-Copper) System[J].Bulletin of Alloy Phase Diagrams, 1982, 3(1): 45
J L Murray, P K Liao, K E Spear. The Ti−B (Titanium-Boron) System[J].Bulletin of Alloy Phase Diagrams, 1986, 7(4): 550
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the Aerospace Innovation Fundation and the Research Fund by the Doctoral Program of High Education (No. 20020213037)
Rights and permissions
About this article
Cite this article
Qiang, X., Xinghong, Z., Jiecai, H. et al. The process of TiB2−Cu composite phase and structure formation during combustion synthesis. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 21, 113–116 (2006). https://doi.org/10.1007/BF02841219
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02841219