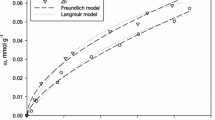
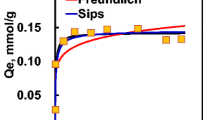
Abstract
Prediction of multicomponent adsorption is still one of the most challenging problems in the adsorption field. Many models have been proposed and employed to obtain multicomponent isotherms from single-component equilibrium data. However, most of these models were based on either unrealistic assumptions or on empirical equations with no apparent definition. The purpose of this investigation was to develop a multicomponent adsorption model based on a thermodynamically consistent equation, and to validate that model using experiment data. Three barbiturates—phenobarbital, mephobarbital, and primidone—were combined to form a ternary system. The adsorption of these barbiturates from simulated intestinal fluid (without pancreatin) by activated carbon was studied using the rotating bottle method. The concentrations, both before and after the attainment of equilibrium, were determined with a high-performance liquid chromatography system employing a reversed-phase column. The proposed equation and the competitive Langmuir-like equation were both fit to the data. A very good correlation was obtained between the experimental data and the calculated data using the proposed equation. The results obtained from the original competitive Langmuir-like model were less satisfactory. These results suggest that the proposed equation can successfully predict the trisolute isotherms of the barbituric acid derivatives employed in this study.
Similar content being viewed by others
Reference
Butler JAV, Ockrent C. Studies in electrocapillarity. Part III. J Phys Chem. 1930;34:2841–2859.
Radke CJ, Prausnitz JM. Thermodynamics of multi-solute adsorption from dilute liquid solutions. AIChE J. 1972;18(4):761–768.
Jossens L, Prausnitz JM, Fritz W, Schlunder EU, Myers AL. Thermodynamics of multisolute adsorption from dilute aqueous solutions. Chem Eng Sci. 1978;33:1097–1106.
Fritz W, Merk W, Schlünder EU, Sontheimer H. In: Suffet IH, McGuire MJ, eds. Activated Carbon Adsorption of Organics from the Aqueous Phase. Vol 1. Ann Arbor, MI: Ann Arbor Science Publishers; 1980:193–195.
Fritz W, Schlünder EU. Competitive adsorption of two dissolved organics onto activated carbon-I. Chem Eng Sci. 1981;36:721–730.
Mckay G, Al Duri B. Prediction of multicomponent adsorption equilibrium data using empirical correlations. Chem Eng J. 1989;41:9–23.
LeVan MD, Vermeulen T. Binary Langmuir-like and Freundlich isotherms for ideal adsorbed solutions. J Phys Chem. 1981;85:3247–3250.
Alkhamis Khouloud A. Prediction of the Adsorption Isotherm Parameters of Various Barbituric Acid Derivatives and Diazepam by Activated Carbon [PhD disseration]. Iowa City, Iowa: University of Iowa; 1997.
Wurster Dale Eric, Alkhamis Khouloud A, Matheson LE. Prediction of adsorption from multicomponent solutions by activated carbon using single-solute parameters. Part I. AAPS PharmSciTech. 2000; 1(3). Artcle 25. www.aapspharmscitech org/scientificjournals/pharmscitech/volumel issue3/031/manuscript.htm
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–1403.
Kipling JJ. Adsorption from Solutions of Non-Electrolytes. New York, NY: Academic Press; 1996:38–39.
Everett DH. Thermodynamics of adsorption from solution. Trans Faraday Soc. 1964;60:1803–1813.
Schwab GM. Theoretische und experimentelle Fortschritte auf dem Gebiete der heterogenen Gasreaktionen. Ergebnisse der Exakten Naturwissenschaften. Vol. 7. Berlin, Germany: Springer, 1928: 276–341.
Markham EC, Benton AF. The adsorption of gas mixtures by silica. J Am Chem Soc. 1931;53:497–506.
Moriguchi I, Kaneniwa N. Adsorption of solute from the solutions. II. Competitive adsorption of cyanocobalamin with pyridoxine and thiamine on talc. Chem Pharm Bull. 1969;17:394–397.
Adamson AW. Adsorption of Gases and Vapors on Solids. In: Physical Chemistry of Surfaces. 6th ed. New York, NY: John Wiley and Sons; 1997:605.
Broughton DB. Adsorption isotherms for binary gas mixtures. Ind and Eng Chem. 1948; 40(8):1506–1508.
Jain JS, Snoeyink VL. Adsorption from bisolute systems on active carbon. J WPCF. 1973;45(12):2463–2479.
Huang L-F. Determination of the Heats of Displacement for Various Barbiturie Acid Derivatives on Two Activated Charcoals [PhD dissertation], Iowa City, Iowa: University of Iowa; 1993.
Burke GM, Wurster Dale Eric, Buraphacheep V, Berg MJ, Veng-Pedersen P, Schottelius, DD. Model selection for the adsorption of phenobarbital by activated charcoal. Pharm Res. 1991;8(2):228–231.
Burke GM. Adsorptivity and Surface Characterization of Activated Charcoals [PhD dissertation]. Iowa City, Iowa: University of Iowa; 1991.
Wurster Dale Eric, Burke GM, Berg MJ, Veng-Pedersen P, Schottelius DD. Phenobarbital adsorption from simulated intestinal fluid, USP, and simulated gastric fluid, USP, by two activated charcoals. Pharm Res. 1988;5(3):183–186.
Bellot JC, Condoret JS. Selection of competitive adsorption model for modelling displacement chromatography. J Chromatogr. 1993;657:305–326.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alkhamis, K.A., Wurster, D.E. Prediction of adsorption from multicomponent solutions by activated carbon using single-solute parameters. Part II—Proposed equation. AAPS PharmSciTech 3, 23 (2002). https://doi.org/10.1208/pt030323
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/pt030323