Abstract
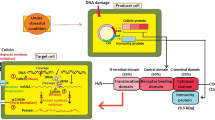
Colicins are toxic exoproteins produced by bacteria of colicinogenic strains ofEscherichia coli and some related species ofEnterobacteriaceae, during the growth of their cultures. They inhibit sensitive bacteria of the same family. About 35%E. coli strains appearing in human intestinal tract are colicinogenic. Synthesis of colicins is coded by genes located on Col plasmids. Until now more than 34 types of colicins have been described, 21 of them in greater detail,viz. colicins A, B, D, E1–E9, Ia, Ib, JS, K, M, N, U, 5, 10. In general, their interaction with sensitive bacteria includes three steps: (1) binding of the colicin molecule to a specific receptor in the bacterial outer membrane; (2) its translocation through the cell envelope; and (3) its lethal interaction with the specific molecular target in the cell. The classification of colicins is based on differences in the molecular events of these three steps.
Similar content being viewed by others
References
Abbott J.D., Graham J.M.: Colicine typing ofShigella sonnei.Month. Bull. Minist. Health Lab. Serv.20, 51–58 (1961).
Abbott J.D., Shannon R.: A new method for typingShigella sonnei using colicin production as a marker.J. Clin. Path.11, 71–77 (1958).
Akutsu A., Masaki H., Ohta T.: Molecular structure and immunity specifity of colicin E6, an evolutionary intermediate between E-group colicins and cloacin DF13.J. Bacteriol.171, 6430–6436 (1989).
Baquero F., Bouanchaud D., Martínez M.C., Perrández C.: Microcin plasmids: a group of extrachromosomal elements coding for low molecular weight antibiotics inEscherichia coli.J. Bacteriol.135, 342–347 (1978).
Baquero F., Moreno F.: The microcins.FEMS Microbiol. Lett.23, 117–124 (1984).
Baty D., Frenette M., Lloubès R., Géli V., Howard S.P., Pattus F., Lazdunski C.: Functional domains of colicin A.Mol. Microbiol.2, 807–811 (1988).
Baty D., Lloubès R., Géli V., Lazdunski C., Howard S.P.: Extracellular release of colicin A is non-specific.EMBO J.6, 2463–2468 (1987).
Bénédetti H., Lloubès R., Lazdunski C., Lettelier L.: Colicin A unfolds during its translocation inEscherichia coli cells and spans the whole cell envelope when its pore has formed.EMBO J.11, 441–447 (1992).
Ben-Gurion R., Hertman I.: Bacteriocin-like material produced byPasteurella pèstis.J. Gen. Microbiol.19, 289–294 (1958).
Bourdineaud J.P., Boulanger P., Lazdunski C., Letellier L.:In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent.Proc. Nat. Acad. Sci. USA87, 1037–1041 (1990).
Bouveret E., Derouiche R., Rigal A., Lloubès R., Lazdunski C., Bénédetti H.: Peptidoglycan-associated lipoprotein-TolB interaction.J. Biol. Chem.270, 11071–11077 (1995).
Bradley D.E.: Colicins G and H and their host strains.Can. J. Microbiol.37, 751–757 (1991).
Bradley D.E., Howard S.P.: A new colicin that adsorbs to the outer membrane protein Tsx but is dependent on thetonB instead of thetolQ membrane transport system.J. Gen. Microbiol.138, 2721–2724 (1992).
Brandis H., Šmarda J.:Bacteriocine und bacteriocinähnliche Substanzen. Gustav Fischer Verlag, Jena 1971.
Braun V.: Covalent lipoprotein from the outer membrane ofEscherichia coli.Biochim. Biophys. Acta415, 335–377 (1975).
Braun V.: Energy-coupled transport and signal transduction through the gram-negative outer membranevia the TonB-ExbB-ExbD dependent receptor proteins.FEMS Microbiol. Rev.16, 295–307 (1995).
Braun V., Günter K., Hantke K.: Transport of iron across the outer membrane.Biol. Met.4, 14–22 (1991).
Braun V., Pilsl H., Gross P.: Colicins: structures, modes of action, transfer through membranes, and evolution.Arch. Microbiol.161, 199–206 (1994).
Cavard D., Lazdunski C.: Colicin cleavage by OmpT protease during both entry into and release fromEscherichia coli cells.J. Bacteriol.172, 648–652 (1990).
Cavard D., Lloubès R., Morlon J., Chartier M., Lazdunski C.: Lysis protein encoded by plasmid ColA-CA31. Gene sequence and export.Mol. Gen. Genet.199, 95–100 (1985).
Chak K.F., Kuo W.S., Lu F.M., James R.: Cloning and characterization of the ColE7 plasmid.J. Gen. Microbiol.137, 91–100 (1991).
Chak K.F., Safo M.K., Ku W.Y., Hsieh S.Y., Yuan H.S.: The crystal structure of the immunity protein of colicin E7 suggests a possible colicin-interacting surface.Proc. Nat. Acad. Sci. USA93, 6437–6442 (1996).
Chan P.T., Ohmori H., Tomizawa J., Lebowitz J.: Nucleotide sequence and gene organization of ColE1 DNA.J. Biol. Chem.260, 8925–8935 (1985).
Clowes R.C.: Colicin factors and episomes.Genet. Res. Cambr.4, 162–165 (1963).
Clowes R.C.: Transmission and elimination of colicin factors and some aspects of immunity to colicin E1 inEscherichia coli.Zbl. Bakt. Hyg. A I Orig.196, 152–160 (1965).
Cole S.T., Saint-Joanis B., Pugsley A.P.: Molecular characterization of the colicin E2 operon and identification of its proteins.Mol Gen. Genet.198, 465–472 (1985).
Cooper P.C., James R.: Two new E colicins, E8 and E9, produced by a strain ofEscherichia coli.J. Gen. Microbiol.130, 209–215 (1984).
Davies J.K., Reeves P.: Genetics of resistance to colicins inEscherichia coli K-12: cross-resistance among colicins of group B.J. Bacteriol.123, 96–101 (1975a).
Davies J.K., Reeves P.: Genetics of resistance to colicins inEscherichia coli K-12: cross-resistance among colinins of group A.J. Bacteriol.123, 102–117 (1975b).
Di Masi D.R., White J.S., Schnaitman C.A., Bradbeer C.: Transport of vitamin B12 inEscherichia coli: common receptor sites for vitamin B12 and E colicins on outer membrane of the cell envelope.J. Bacteriol.115, 506–513 (1973).
Drury L.S., Buxton R.S.: Identification and sequencing of theEscherichia coli cet gene which codes for an inner membrane protein, mutation of which causes tolerance to colicin E2.Mol. Microbiol.2, 109–119 (1988).
Duché D., Izard J., Gonzáles-Mañas J.M., Parker M.W., Crest M., Chartier M., Baty D.: Membrane topology of the colicin A pore-forming domain analyzed by disulfide bond engineering.J. Biol. Chem.271, 15401–15406 (1996).
Duché D., Letellier L., Géli V., Bénédetti H., Baty D.: Quantification of group A colicin import sites.J. Bacteriol.177, 4935–4939 (1995).
El Kouhen R., Hoenger A., Engel A., Pages J.:In vitro approaches to investigation of the early steps of colicin-OmpF interaction.Eur. J. Biochem224, 723–728 (1994).
van den Elzen P.J.M., Walters H.H.B., Veltkamp E., Nukamp H.J.: Molecular structure and function of the bacteriocin gene and bacteriocin protein of plasmid cloDF13.Nucl. Acids. Res.11, 2465–2477 (1983).
Eraso J.M., Chidambaram M., Weinstock G.M.: Increased production of colicin E1 in stationary phase.J. Bacteriol.178, 1928–1935 (1996).
Eraso J.M., Weinstock G.M.: Anaerobic control of colicin E1 production.J. Bacteriol.174, 5101–5109 (1992).
Espesset D., Duché D., Baty D., Géli V.: The channel domain of colicin A is inhibited by its immunity protein through direct interaction in theEscherichia coli inner membrane.EMBO J.15, 2356–2364 (1996).
Evans L.J.A., Cooper A., Lakey J.H.: Direct measurement of the association of a protein with a family of membrane receptors.J. Mol. Biol.255, 559–563 (1996).
Ferber D.M., Brubaker R.R.: Mode of action of pesticin: N-acetylglucosaminidase activity.J. Bacteriol.139, 495–501 (1979).
Ferber D.M., Fowler J.M., Brubaker R.R.: Mutations to tolerance and resistance to pesticin and colicins inEscherichia coli.J. Bacteriol.146, 506–511 (1981).
Foulds J., Shemin D.: Properties and characteristics of a bacteriocin fromSerratia marcescens.J. Bacteriol.99, 655–660 (1969).
Fredericq P.: Sur la pluralité des récepteurs d'antibiose d'E. coli.Compt. Rend. Soc. Biol.140, 1189–1190 (1946).
Fredericq P.: Actions antibiotiques réciproques chez lesEnterobacteriaceae.Rev. belge Path. exp. Med. exp.19, suppl. 4, 1–107 (1948).
Fredericq P.: Acquisition de proprietes antibiotiques nouvelles par la soucheE. coli V sous l'action des bacteriophages T.1, T.5 et T.7.Antonie van Leeuwenhoek J. Microbiol. Serol.17, 102–106 (1951).
Fredericq P.: A note on the classifications of colicins.Zbl. Bakt. Hyg. A I Orig.196, 140–142 (1965).
Fredericq P., Betz-Bareau M.: Transfert génétique de la propriété colicinogène chezE. coli.Compt. Rend. Soc. Biol.147, 1110–1112 (1953).
Fredericq P., Joiris E., Betz-Bareau M., Gratia A.: Recherche des germes producteurs de colicines dans les selles de malades atteints de fièvre paratyphoide B.Compt. Rend. Soc. Biol.143, 556–559 (1949).
Fredericq P., Šmarda J.: Complexite du facteur colicinogène B.Ann. Inst. Pasteur118, 767–774 (1970).
Gardner J.F.: Some antibiotics formed byBacterium coli.Brit. J. exp. Path.31, 102–111 (1950).
Géli V., Lazdunski C.: An α-helical hydrophobic hairpin as a specific determinant in protein-protein interaction occurring inEscherichia coli colicin A and B immunity systems.J. Bacteriol.174, 6432–6437 (1992).
Gratia A.: Sur un remarquable exemple d'anatagonisme entre deux souches de colibacille.Comp. Rend. Soc. Biol.93, 1040–1041 (1925).
Gratia A.: Antagonisme bactérien et bactériophagie.Ann. Inst. Pasteur48, 113–137 (1932).
Gratia A., Fredericq P.: Diversité des souches antibiotiques deB. coli et étendue variable de leurs champs d'action.Comp. Rend. Soc. Biol.140, 1032–1033 (1946).
Gross P., Braun V.: Colicin M is inactivated during import by its immunity protein.Mol. Gen. Genet.251, 388–396 (1996).
Guasch J.F., Enfedaque E., Ferrer S., Gargallo D., Regué M.: Bacteriocin 28b, a chromosomally encoded bacteriocin produced by mostSerratia marcescens biotypes.Res. Microbiol.146, 447–483 (1995).
Guihard G., Boulanger P., Bénédetti H., Lloubès R., Besnard M., Lettelier L.: Colicin A and the Tol proteins involved in its translocation are preferentially located in the contact sites between the inner and outer membranes ofEscherichia coli cells.J. Biol. Chem.269, 5874–5880 (1994).
Hamon Y., Péron Y.: A propos de quelques nouveaux types de colicines thermostables.Compt. Rend. Acad. Sci.258, 3121–3124 (1964a).
Hamon Y., Péron Y.: Description de sept nouveaux types de colicines. État actuel de la classification de ces antibiotiques.Ann. Inst. Pasteur106, 44–54 (1964b).
Hantke K., Braun V.: Membrane receptor dependent iron transport inEscherichia coli.FEBS Lett.49, 301–305 (1975).
Hauduroy P., Papavassiliou J.: Identification of a new type of colicine (colicine L).Nature195, 730–732 (1962).
Hejátko J., Šmarda J.: Temporary desorption of colicin during the process of its adsorption on sensitive bacteria.Scripta med. (Brmo), in press (1998).
Hill C., Holland I.B.: Genetic basis of colicin E susceptibility inEscherichia coli. I. Isolation and properties of refractory mutants and the preliminary mapping of their mutations.J. Bacteriol.94, 677–686 (1967).
Hirose A., Kumagai J., Ihamori K.: Dissociation and reconstitution of colicin E3 and immunity substance complex.J. Biochem. (Tokyo)79, 305–311 (1976).
Horák V.: Two new colicins fromShigellae.Fol. Microbiol.35, 469–470 (1990).
Horák V.: Seventy colicin types ofShigella sonnei and an indicator system for their determination.Zbl. Bakt. Hyg. A I Orig.281, 24–29 (1994).
Horák V., Sobotková J.: Sensitivity to colicin JS, one of important characteristics ofEscherichia coli strains belonging to enteroinvasive serovars.Zbl. Bakt. Hyg. A I Orig.269, 156–159 (1988).
Jacob F., Siminovitch L., Wollman É.: Sur la biosynthèse d'une colicine et sur son mode d'action.Ann. Inst. Pasteur83, 295–315 (1952).
Jakes K.S., Davis N.G., Zinder N.D.: A hybrid toxin from bacteriophage f1 attachment protein and colicin E3 has altered cell receptor specifity.J. Bacteriol.170, 4231–4238 (1988).
Jakes K.S., Zinder N.: Highly purified colicin E3 contains immunity protein.Proc. Nat. Acad. Sci. USA71, 3380–3384 (1974).
James R., Jarvis M., Barker D.F.: Nucleotide sequence of the immunity and lysis region of the ColE9-J plasmid.J. Gen. Microbiol.133, 1553–1562 (1987).
James R., Kleanthous C., Moore G.R.: The biology of E-colicins: paradigms and paradoxes.Microbiol.142, 1569–1580 (1996).
Jeanteur D., Schirmer T., Fourel D., Simonet V., Rummel G., Widmer C., Rosenbusch J.P., Pattus F., Pages J.: Structural and functional altertions of a colicin-resistant mutant of OmpF-porin fromEscherichia coli Proc. Nat. Acad. Sci. USA91, 10675–10679 (1994).
Kadner R.J.: Vitamin B12 transport inEscherichia coli: energy coupling between membranes.Mol. Microbiol.4, 2027–2033 (1990).
Kadner R.J., Bassford P.J. Jr.,Pugsley A.P.: Colicin receptors and mechanisms of colicin uptake.Zbl. Bakt. Hyg. A I Orig.244 90–104 (1979).
Kageyama M., Kobayashi M., Sano Y., Masaki H.: Construction and characterization of pyocin-colicin chimeric proteins.J. Bacteriol.178, 103–110 (1996).
Konisky J.: The Bacteriocins, pp. 71–136 inThe Bacteria: a Treatise on Structure and Function, Vol. 6Bacterial Diversity (L.N. Ornston, J.R. Sokatch, Eds.) Academic Press, New York 1978.
Konisky J., Nomura M.: Interaction of colicins with bacterial cells. II. Specific alteration ofEscherichia coli ribosomes induced by colicin E3in vivo.J. Mol. Biol.26, 181–195 (1967).
Konisky J., Richards F.M.: Characterization of colicin Ia and Ib. Purification and some physical properties.J. Biol. Chem.245, 2972–2978 (1970).
Lasater L.S., Cann P.A., Glitz D.G.: Localization of the site of cleavage of ribosomal RNA by colicin E3. Placement of the small ribosomal subunit by electron microscopy of antibody-complementary oligodeoxynucleotide complexes.J. Biol. Chem.264, 21798–21805 (1989).
Lau P.C.K., Condie J.A.: Nucleotide sequences from the colicins E6 and E9 operons: presence of a degenerate transposon-like structure in the ColE9-J plasmid.Mol. Gen. Genet.217, 269–277 (1989).
Lazdunski C.: Colicin import and pore-formation: a system for studying protein transport across membranes?Mol. Microbiol.16, 1059–1066 (1995).
Lazdunski C., Baty D., Géli V., Cavard D., Morlon J., Lloubès J., Howard S.P., Knibiehler M., Chartier M., Varenne S., Frenette M., Dasseux J.L. Pattus F.: The membrane channel-forming colicin A: synthesis, secretion, structure, action and immunity.Biochim. Biophys. Acta947, 4445–4464 (1988).
Lazzaroni J.C., Vianney A., Popot J.L., Bénédetti H., Samatey F., Lazdunski C., Portalier R., Géli V.: Transmembrane α-helix interactions are required for the functional assembly of theEscherichia coli Tol complex.J. Mol. Biol.246, 1–7 (1995).
Lokaj J., Šmarda J., Mach J.: Colicin E3 enhances the oxidoreductive activity of guinea-pig leukocytes.Experientia38, 1352–1353 (1982).
Lotz W.: Effect of guanosine tetraphosphate onin vitro protein synthesis directed by E1 and E3 colicinogenic factors.J. Bacteriol.135, 707–712 (1978).
Lu F.M., Chak K.F.: Two overlapping SOS boxes in ColE1 operon are responsible for the viability of cells harboring the Col plasmid.Mol. Gen. Genet.251, 407–411 (1996).
Luirink J., Mol O., Oudega B.: Functioning of the pColDF13 encoded BRP, pp. 307–316 inBacteriocins, Microcins and Lantibiotics (R. James, C. Lazdunski, F. Pattus, Eds). Springer-Verlag, Berlin-Heidelberg-New York 1992.
Males B.M., Stocker B.A.D.:Escherichia coli K317, formerly used to define colicin group E2, produces colicin E7, is immune to colicin E2, and carries a bacteriophage-restricting conjugative plasmid.J. Bacteriol.144, 524–531 (1980).
Males B.M., Stocker B.A.D.: Colicin E4, colicin E5, colicin E6 and colicin A and properties ofbtub+ colicinogenic transconjugants.J. Gen. Microbiol.128, 95–106 (1982).
Mathildah M.T., Timmis K.N., Diaz E.: Use of colicin E3 for biological containment of microorganisms.Appl. Environ. Microbiol.62, 1805–1807 (1996).
Matsuzawa H., Ushiyama S., Koyama Y., Ohta T.:Escherichia coli K-12tolZ mutants tolerant to colicins E2, E3, D, Ia and Ib: defect in generation of the electrochemical proton gradient.J. Bacteriol.160, 733–739 (1984).
Mock M., Pugsley A.P.: The BtuB group Col plasmids and homology between the colicins they encode.J. Bacteriol.150, 1069–1076 (1982).
Morlon J., Lloubès R., Varenne S., Chartier M., Lazdunski C.: Complex nucleotide sequence of the structural gene for the colicin A, a gene translated at a non-uniform rate.J. Mol. Biol.170, 271–285 (1983).
Nagel de Zwaig R., Luria S.E.: Genetics and physiology of colicin-tolerant mutants ofEscherichia coli.J. Bacteriol.94, 1112–1123 (1967).
Nomura M.: Mode of action of colicins.Cold Spring Harbor Symp. Quant. Biol.28, 315–324 (1963).
Nomura M.: Mechanism of action of colicines.Proc. Nat. Acad. Sci. USA52, 1514–1521 (1964).
Nomura M., Nakamura M.: Reversibility of inhibition of nucleic acids and protein synthesis by colicin K.Biochem. Biophys. Res. Comm.7, 306–309 (1962).
Nomura M., Witten C.: Interaction of colicins with bacterial cells. III. Colicin-tolerant mutations inEscherichia coli.J. Bacteriol.94, 1093–1111 (1967).
Ölschläger T., Braun V.: Sequence, expression and localization of the immunity protein for colicin M.J. Bacteriol.169, 4765–4769 (1987).
Ölschläger T., Turba A., Braun V.: Binding of the immunity protein inactivates colicin M.Mol. Microbiol.5, 1105–1111 (1991).
Papavassiliou J.: Biological characteristics of colicine X.Nature160, 110 (1961).
Parker M.W., Pattus F., Tucker A.D., Tsernoglou D.: Structure of the membrane-pore-forming fragment of colicin A.Nature337, 93–96 (1989).
Pattus F., Massote D., Wilmsen H.U., Lakey J., Tsernoglou D., Tucker A., Parker M.W.: Colicins: prokaryotic killer pores.Experientia46, 181–191 (1990).
Pilsl H., Braun V.: Novel colicin 10: assignment of four domains to TonB- and TolC-dependent uptakevia the Tsx receptor and to pore formation.Mol. Microbiol.16, 57–67 (1995a).
Pilsl H., Braun V.: Evidence that the immunity protein inactivates colicin 5 immediately prior formation of the transmembrane channel.J. Bacteriol.177, 6966–6972 (1995b).
Pilsl H., Braun V.: Strong function-related homology between the pore-forming colicins K and 5.J. Bacteriol.177, 6973–6977 (1995c).
Pilsl H., Killmann H., Hantke K., Braun V.: Periplasmic location of the pesticin immunity protein suggest inactivation of pesticin in the periplasm.J. Bacteriol.178, 2431–2435 (1996).
Pugsley A.P.: The ins and outs of colicins. I. Production, and translocation across membranes.Microbiol. Sci.1, 168–175 (1984a).
Pugsley A.P.: The ins and outs of colicins. II. Lethal action, immunity and ecological implications.Microbiol. Sci.1, 203–205 (1984b).
Pugsley A.P.: Nucleotide sequencing of the structural gene for colicin N reveals homology between the catalytic C-terminal domains of colicin A and colicin N.Mol. Microbiol.1, 317–325 (1987).
Pugsley A.P.: The immunity and lysis genes of ColN plasmid pCHAP4.Mol. Gen. Genet.211, 335–341 (1988).
Pugsley A.P., Schwartz M.: A genetic approach to the study of mitomycin-induced lysis ofEscherichia coli K-12 strains which produce colicin E2.Mol. Gen. Genet.190, 366–372 (1983).
Pugsley A.P., Schwartz M.: Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase.EMBO J.3, 2393–2397 (1984).
Qui X.Q., Jakes K.S., Kienker P.K., Finkelstein A., Slatin S.L.: Major transmembrane movement associated with colicin Ia channel gating.J. Gen. Physiol.107, 313–328 (1996).
Reeves P.: The bacteriocins.Bacteriol. Rev.29, 24–45 (1965).
Reeves P.:The Bacteriocins. Springer-Verlag, Berlin-Heidelberg-New York 1972.
Roos U., Harkness R.E., Braun V.: Assembly of colicin genes from a few DNA fragments. Nucleotide sequence of colicin D.Mol. Microbiol.3, 891–902 (1989).
Sagik J.F., Suit J.L., Luria S.E.:cea-kil operon of the ColE1 plasmid.J. Bacteriol.153, 1479–1485 (1983).
Salles B., Weisemann J.M., Weinstock G.M.: Temporal control of colicin E1 induction.J. Bacteriol.169, 5028–5034 (1987).
San Millán J.L., Kolter R., Moreno F.: Evidence that colicin X is microcin B17.J. Bacteriol.169, 2899–2901 (1987).
Schaller K., Holtje J.-V., Braun V.: Colicin M is an inhibitor of murein synthesis.J. Bacteriol.152, 994–1000 (1982).
Schaller K., Krauel A., Braun V.: Temperature sensitive, colicin M-tolerant mutant ofEscherichia coli.J. Bacteriol.147, 135–139 (1981).
Schramm E., Mende J., Braun V., Kamp R.M.: Nucleotide sequence of colicin B activity genecba: consensus peptapeptide among TonB-dependent colicins and receptors.J. Bacteriol.169, 3350–3357 (1987).
Šmajs D.: The morphology of bacterial cell in inhibition zones produced by colicins.Scripta med. (Brno)68, 171–180 (1995).
Šmajs D., Pisl, H., Braun V.: Colicin U, a novel colicin produced byShigella boydii.J. Bacteriol.179, 4919–4928 (1997).
Šmarda J.: Incidence and manifestations of colicinogeny in strains ofEscherichia coli.J. Hyg. Epid. Microbiol. Immunol.4, 151–165 (1960).
Šmarda J.: Some problems of the immediate action of colicines on susceptible bacteria.Antimicrob. Agents Chemother. 345–348 (1965).
Šmarda J.: Novel approaches to the mode of action of colicins.Fol. Microbiol.20, 264–271 (1975).
Šmarda J.: The action of colicins on eukaryotic cells.J. Toxicol. Toxin Rev.2, 1–76 (1983).
Šmarda J.: Production of bacteriocin-like agents ofBudvicia aquatica and “Pragia fontium”.Zbl. Bakt. Hyg. A I Orig.265, 74–81 (1987).
Šmarda J.: Resistance and tolerance of bacteria to E colicins, pp. 493–502 inBacteriocins, Microcins and Lantibiotics (R. James, C. Lazdunski, F. Pattus, Eds). Springer-Verlag, Berlin-Heidelberg-New York 1992a.
Šmarda J.: Colicins as anti-tumour drugs, pp. 505–510 inBacteriocins, Microcins and Lantibiotics (R. James, C. Lazdunski, F. Pattus, Eds). Springer-Verlag, Berlin-Heidelberg-New York 1992b.
Šmarda J., Damborský J.: A quantitative assay of E group colicins.Scripta med. (Brno)64, 111–118 (1991).
Šmarda J., Obdržálek V.: Colicine Q.Zbl. Bakt. Hyg. A I Orig.200, 493–497 (1966).
Šmarda J., Obdržálek V.: The lethal effect of colicin E3 on HeLa cells in tissue cultures.IRCS J. Med. Sci.5, 524 (1977).
Šmarda J., Obdržálek V., Táborský I., Mach J.: The cytotoxic effect of colicin E3 on mammalian tissue cells.Fol. Microbiol.23, 272–277 (1978).
Šmarda J., Oravec C.:In vitro andin vivo inhibition of blast lymphocytes by colicins.Fol. Microbiol.38, 120 (1993).
Šmarda J., Petrželová J., Vyskot B.: Colicin JS ofShigella sonneii: classification of type colicin “7”.Zbl. Bakt. Hyg. A I Orig.263, 530–540 (1987).
Šmarda J., Schuhmann E.: Studies of colicin action on wall-less stable L-forms ofEscherichia coli. I. Degree of attachment and of killing effect on rods and stable L-form cells.Z. Allg. Mikrobiol.19, 511–516 (1979).
Šmarda J., Ševčíková I.: Mutation analysis of the receptor for colicins E1–E7. A pilot study.Fol. Microbiol.33, 59–67 (1988).
Šmarda J., Šmarda J., Jr.,Vrbická Z.: Colicins E7 and E8 degrade DNA in sensitive bacteria.Fol. Microbiol.35, 348–352 (1990).
Šmarda J., Taubeneck U.: Situation of colicin receptors in surface layers of bacterial cells.J. Gen. Microbiol.52, 161–172 (1968).
Song H.Y., Cramer W.A.: Membrane topography of ColE1 gene products: the immunity protein.J. Bacteriol.173, 2935–2943 (1991).
Sonnenborn U., Greinwald R.:Beziehungen zwischen Wirtorganismus und Darmflora. Schattauer, Stuttgart-New York 1991.
Stegehius F., van der Wal F.J., Luirink J., Oudega B.: Expression of the pColDF13 encoded bacteriocin release protein or its stable signal peptide causes early effects on protein biosynthesis and Mg2+ transport.Antonie van Leeuwenhoek J. Microbiol. Serol.67, 255–260 (1995).
Stouthamer A.H., Tietze G.A.: Bacteriocin production by members of the genusKlebsiella.Antonie van Leeuwenhoek J. Microbiol. Serol.32, 171–182 (1966).
Suzuki H.: Colicin E3 inhibits rabbit globin synthesis.FEBS Lett.89, 121–125 (1978).
Tatsumi Y., Maejima T., Mitsuhashi S.: Mechanism oftonB-dependent transport of KP736, a 1,5-dihydroxy-4-pyridone-substituted cephalosporin, intoEscherichia coli K-12 cells.Antimicrob. Agents. Chemother.39, 613–619 (1995).
Threlfall E.J., Holland I.B.: Co-transduction with SerB of a pleiotropic mutation affecting colicin E2 refractivity, ultraviolet sensitivity, recombination proficiency and surface properties ofEscherichia coli K12.J. Gen. Microbiol.62, 383–398 (1970).
Toba M., Masaki H., Ohta T.: Colicin E8, a DNase which indicates an evolutionary relationship between colicins E2 and E3.J. Bacteriol.170, 3237–3242 (1988).
Uratani Y., Cramer W.A.: Reconstitution of colicin E1 into dimyristoylphosphatidylcholine membrane vesicles.J. Biol. Chem.256, 4017–4023 (1981).
Viejo M.B., Enfedaque J., Guasch J.F., Ferrer S., Regué M.: Protection against bacteriocin 28b inSerratia marcescens is apparently not related to the expression of an immunity gene.Can. J. Microbiol.41, 217–226 (1995).
Viejo M.B., Gargallo D., Ferrer S., Enfedaque J., Regué M.: Cloning and DNA sequence analysis of a bacteriocin gene fromSerratia marcescens.J. Gen. Microbiol.138, 1737–1743 (1992).
Viklický V., Šmarda J., Dráber Petr, Pokorná Z., Mach J., Dráber Pavel: The cytoplasmic membrane as a site of the primary effect of colicin on eucaryotic cells.Folia Biol. (Prague)25, 116–125 (1979).
van der Wal F.J., ten Hagen C.N., Oudega B., Luirink J.: Application of the pColDF13 bacteriocin release protein in the release of the heterologous proteins ofEscherichia coli: production of plant α-galactosidase.Biotechnol. Lett.17, 815–820 (1995a).
van der Wal F.J., Luirink J., Oudega B.: Bacteriocin release proteins: mode of action, structure and biotechnological application.FEMS Microbiol. Rev.17, 381–399 (1995b).
Vollmer W., Pilsl H., Hantke K., Holtje J.-V., Braun V.: Pesticin displays muramidase activity.J. Bacteriol.179, 1580–1583 (1977).
Whielan K.F., Colleran E., Taylor D.E.: Phage inhibition, colicin resistance, and tellurite resistance are encoded by a single cluster of genes on the IncHI2 plasmid R478.J. Bacteriol.177, 5016–5027 (1995).
Wooldridge K.G., Williams P.H.: Sensitivity ofEscherichia coli to cloacin DF13 involves the major outer membrane protein OmpF.J. Bacteriol.173, 2420–2424 (1991).
Author information
Authors and Affiliations
Additional information
The original version of this review was published in Czech in the journal “Biologické listy”,62, 107–130 (1997).
Rights and permissions
About this article
Cite this article
Šmarda, J., Šmajs, D. Colicins—Exocellular lethal proteins ofEscherichia coli . Folia Microbiol 43, 563–582 (1998). https://doi.org/10.1007/BF02816372
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02816372