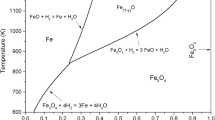
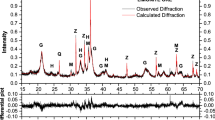
Abstract
The pore structure of iron reduced from hematite ore by hydrogen is charactertized from measurements of pore volume, pore area and effective gas diffusivity. The measured connected pore volume within the range 0.22 to 0.34 cu cm per g is about the same as the total pore volume; that is, most of the pores in the reduced iron are interconnected. The pore area measured by the BET technique increases with decreasing reduction temperature,e.g. from 0.1 sq m per g at 1200°C to 39 sq m per g at 200°C. The effective H2−H2O diffusivity, measured directly at 600°C after reduction of the ore in hydrogen at the same temperature, is in excellent agreement with that derived from the reduction data. The pore-diffusivity measurements were also made at room temperature using iron samples reduced in hydrogen at 800° and 1000°C. On the basis of the pore properties measured, it appears that the reduced iron has a regular pore structure which becomes finer with decreasing reduction temperature. The effective diffusivities computed on the basis of a simple pore structure are found to be in accord with those derived previously from the reduction data.
Similar content being viewed by others
References
E. T. Turkdogan and J. V. Vinters:Met. Trans., 1971, vol. 2, pp. 3175–88.
E. T. Turkdogan, R. G. Olsson, and J. V. Vinters:Carbon, 1970, vol. 8, pp. 545–64.
R. G. Olsson and W. M. McKewan:Trans. TMS-AIME, 1966, vol. 236, pp. 1518–22:Met. Trans., 1970, vol. 1, pp. 1507–12.
W. M. McKewan:Steelmaking, The Chipman Conference, pp. 141–55, MIT Press, Massachusetts, 1965.
W. G. Pollard and R. D. Present:Phys. Rev., 1948, vol. 73, pp. 762–74.
C. H. Bosanquet: British TA Report BR-507, September 27, 1944.
R. B. Evans III, G. M. Watson, and E. A. Mason:J. Chem. Phys., 1961, vol. 35, pp. 2076–83.J. Chem. Phys., 1962, vol. 36, pp. 1894–1902.
D. S. Scott and F. A. Dullien:Am. Inst. Chem. Engr. J., 1962, vol. 8, pp. 113–17.
L. B. Rothfeld:Am. Inst. Chem. Engr. J., 1963, vol. 9, pp. 19–24.
J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird:Molecular Theory of Gasses and Liquids, John Wiley & Sons, Inc., 1954.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Turkdogan, E.T., Olsson, R.G. & Vinters, J.V. Gaseous reduction of iron oxides: Part II. Pore characteristics of iron reduced from hematite in hydrogen. Metall Trans 2, 3189–3196 (1971). https://doi.org/10.1007/BF02814971
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02814971