Summary
Conclusion
Inl-arginine (Arg)-induced pancreatitis, evidence of acute inflammation was observed on d 1–3. Continuous tissue atrophy became visible at the sites of previous pancreatic necrosis, with simultaneous regeneration of the pancreas, mainly around the Langerhans islets. Administration of low doses of cholecystokinin-octapeptide (CCK-8) increased the inflammatory signs of pancreatitis in the early phase, but subsequently diminished the level of atrophy and accelerated the processes of regeneration in this model of pancreatitis.
Background
The aim of this work was to study the regenerative processes following Arg-induced pancreatitis in rats. Besides the spontaneous regeneration, the effects of low doses of CCK-8 on the laboratory and morphologic parameters in this type of experimental pancreatitis were investigated.
Methods
Male Wistar rats were divided into three groups. In group I, the rats received 200 mg/100 g body weight of Arg ip twice, at an interval of 1 h, and 0.5 mL saline was administered sc twice daily. In group II, besides the same amount of Arg, the rats received 1 μg/kg of CCK-8 sc in 0.5-mL saline twice daily (7am and 7pm). In the control animals (group III), an identical amount of glycine was administered ip instead of Arg at the same times. The rats were examined on d 1, 3, 7, 14, and 28 after pancreatitis induction. The pancreatic weight/body weight ratio (pw/bw) was calculated in each case. The serum levels of amylase, and glucose and the pancreatic contents of soluble protein, trypsin, amylase and DNA were determined, and histologic examinations were performed.
Results
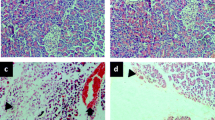
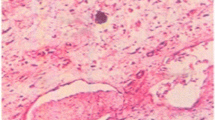
In groups I and II, both pw/bw (3.5±0.2 mg/g and 4.1±0.28 mg/g, respectively) and the serum amylase level (8900±560 IU/L and 11100±1390 IU/L, respectively) were significantly elevated on d 1 vs group III (2.1±0.06 mg/g and 5562±373 IU/L, respectively). Pw/bw was significantly decreased in groups I (0.96±0.12 mg/g, 0.8±0.1 mg/g, and 1.8±0.1 mg/g, respectively) and II (1.4±0.15 mg/g, 1.7±0.2 mg/g, and 1.95±0.1 mg/g, respectively) on d 7, 14, and 28 vs group III (2.6±0.3 mg/g, 3.1±0.15 mg/g, and 2.7 ±0.1 mg/g, respectively), whereas in group II it was significantly elevated vs. group I on d 7 and 14. The pancreatic contents of soluble protein, DNA, trypsin and amylase were significantly decreased on d 3–14 in groups I and II vs group III. The pancreatic DNA level was significantly elevated in group II (1.23±0.2 mg/pancreas) vs group I (0.7±0.1 mg/pancreas) on d 7. In group II, the soluble protein (73.1±15.5 mg/pancreas) and amylase (1104±160 IU/pancreas) levels were significantly elevated on d 14 as was that of trypsin (27.2±3.1 IU/pancreas) on d 28, vs group I (26.4±5.3 mg/p, 525±111 IU/pancreas, and 16.3±1.1 IU/pancreas, respectively). On histologic sections, the signs of acute inflammation of the pancreas were more pronounced in group II than in group I on d 1–3. After that time, in spite of the progressive atrophy of the pancreas, the signs of tissue repair were more expressed in group II.
Similar content being viewed by others
References
Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas.J Nutr 1984; 114: 467–471.
Kishino Y, Kawamura S. Pancreatic damage induced by injecting a large dose of arginine.Wirchows Archiv (Cell Pathol) 1984; 47: 149–155.
Tani S, Itoh H, Okabayashi Y, Nakamura T, Fujii M, Fujisawa T, Koide M, Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats.Dig Dis Sci 1990; 35: 367–374.
Delaney CP, McGeeney KF, Dervan P, Fitzpatrick JM. Pancreatic atrophy: A new model using serial intraperitoneal injections of L-arginine.Scand J Gastroenterol 1993; 28: 1086–1090.
Takács T, Nagy I, Pap À, Varró V. Effect of a new CCK receptor antagonist on pancreatic secretion and groth.Digestion 1987; 38: 60.
Solomon TE. Regulation of exocrine pancreatic cell proliferation and enzyme synthesis, inPhysiology of Gastrointestinal Tract, Johnson LR, ed. Raven, New York, 1989; pp. 873–892.
Pap À, Boros L, Hajnal F. Essential role of cholecystokinin in pancreatic regeneration after 60% distal resection in rats.Pancreas 1991; 6: 412–418.
Takács T, Nagy I, Pap À, Varró V. The effect of long-term administration of lorglumide (CR 1409) on rat pancreatic growth and enzyme composition.Pancreas 1990; 5: 606–610.
Lászik GZ, Berger Z, Pap À, Tóth KG, Varró V. Course and regression of acute interstitial pancreatitis induced in rats by repeated serial subcutaneous cholecystokinin-oktapeptide injections.Int J Pancreatol 1989; 5: 347–358.
Tani S, Itoh H, Koide M, Okabayasi Y, Otsuki M. Involvement of endogenus cholecystokinin in the development of acute pancreatitis induced by closed duodenal loop.Pancreas 1993; 8: 109–115.
Jurkowska G, Grondin G, Morisset J. Involvement of endogenous cholecystokinin in pancreatic regeneration after cerulein-induced acute pancreatitis.Pancreas 1992; 7: 295–304.
Ceska M, Birath K, Brown B. A new and rapid method for the clinical determination of a-amylase activates in human serum and urine.Clin Chim Acta 1969; 26: 437–444.
Hyvärinen A, Nikkilä EA. Specific determination of blood glucose with o-toluidine.Clin Chim Acta 1962; 7: 140–144.
Schneider WC. Determination of nucleic acids in tissues by pentose analysis.Meth Enzymol 1957; 3: 680–684.
Giles KW, Myers A. An improved diphenylamine method for the estimation of deoxyribonucleic acid.Nature (UK) 1965; 206: 93.
Nagy I, Pap À, Varró V. Time-course of changes in pancreatic size and enzyme composition in rats during starvation.Int J Pancreatol 1989; 5: 35–45.
Solomon TE, Petersen H, Elashoff J, Grossman MT. Interaction of caerulein and secretin on pancreatic size and composition in rat.Am J Physol 1978; 235: E714-E719.
Dagorn JC. Nonparallel enzyme secretion from the rat pancreas: in vivo studies.J Physiol (UK) 1978; 280: 435–448.
Erlanger BF, Kokowsky W, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin.Arch Biochem Biophys 1961; 95: 271–278.
Goa J. Micro biuret method for protein determination; determination of total protein in cerebrospinal fluid.Scand J Clin Lab Invest 1953; 5: 218–222.
Odaira C, Berger Z, Iovanna JL, Sarles H. Localized necrohemorrhagic pancreatitis in the rat after pancreatic interstitial trypsin Injection.Digestion 1986; 34: 68–77.
Waever C, Bishop AE, Polak JM. Pancreatic changes elicited by chronic administration of excess L-arginine.Exp Mol Pathol 1994; 60: 71–87.
Scheele G. Regulation of gene expression in the exocrine pancreas, inThe Exocrine Pancreas, Go VL, Gardner JD, Brooks FP, Lebenthal E, Di Magno EP, Scheele GA, eds. Raven, New York, 1986; pp. 55–69.
Yanatory Y, Fujita T. Hypertrophy and hyperplasia in the endocrine and exocrine pancreas of rats fed soyben trypsin inhibitor or repeatedly injected with pancreozymin.Arch Histol Jpn 1976; 39: 67–78.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hegyi, P., Takács, T., Jármay, K. et al. Spontaneous and cholecystokinin-octapeptide-promoted regeneration of the pancreas followingl-arginine-induced pancreatitis in rat. Int J Pancreatol 22, 193–200 (1997). https://doi.org/10.1007/BF02788384
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02788384