Abstract
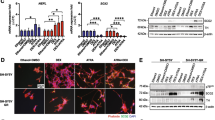
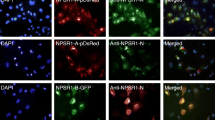
Activating mutations of the receptor tyrosine kinase,ret, are associated with multiple endocrine neoplasia type 2A (MEN-2A). However, the mechanisms leading to tumor development are unclear. Glial-derived neurotrophic factor (GDNF) activates wild-typeret via interaction with a second receptor, GFR α-1. We have utilized GDNF to stimulate normal and neoplastic chromaffin cells in order to ask whetherret activation is mitogenic. Cells from three normal adult adrenal medullas, one sporadic pheochromocytoma, and three MEN-2A pheochromocytomas were labeled with bromodeoxyuridine (BrdU) for 12 d in the presence or absence of GDNF or nerve growth factor (NGF), which is known to stimulate neurite outgrowth, but not proliferation in human chromaffin and pheochromocytoma cell cultures. Responses to GDNF and NGF were comparable, except for two MEN-2A pheochromocytomas that responded minimally to GDNF and robustly to NGF. These tumors responded to GDNF biochemically, as measured by phosphorylation of mitogen-activated protein kineses, despite their weak morphological responses. Our findings suggest that activation ofret may not be sufficient to produce chromaffin cell hyperplasia or neoplasia directly by stimulating cell proliferation. However, the possibility that altered cell-cell or cell-substrate interactions might cause responses to become differentiative rather than proliferative in vitro has not been ruled out. We also demonstrate, for the first time, that at least some human pheochromocytomas with an MEN-2Aret mutation respond to a normalret ligand. This responsiveness could be mediated by a remaining normalret allele or by other mechanisms.
Similar content being viewed by others
References
Ponder BAJ, Piero MA. Mutations in ret in MEN 2. In: Nelkin BD, Austin, RG, eds. Genetic Mechanisms in Multiple Endocrine Neoplasia Type 2. Landes, Austin, TX 1996; 21–35.
Wada M, Asai N, Tsuzuki T, Maruyama S, Ohiwa M, Imai T, et al. Detection ofret homodimers in MEN 2A-associated pheochromocytomas. Biochem Biophys Res Commun 218:606–609, 1996.
Trupp M, Arenas E, Fainzilber M, Nilsson A-S, Sieber B-A, Grigoriou M, et al. Functional receptor for GDNF encoded by the c-ret protooncogene. Nature 381:785–788, 1996.
Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, et al. Characterization of a multicomponent receptor for GDNF. Nature 382:80–83, 1996.
GFRα Nomenclature Committee. Nomenclature of GPI-linked receptors for the GDNF ligand family. Neuron 19:485, 1997.
Fusco A, Vecchio G, Dathan NA, Carlomagno F, DiFiore PP, Santoro M. Intracellular signalling by theret tyrosine kinase. In: Nelkin BD, ed. Genetic Mechanisms in, Multiple Endocrine Neoplasia Type 2. Landes, Austin, TX 1996; 37–59.
Gagel R, Tashjian A Jr, Cummings T. The clinical outcome of prospective screening for Multiple Endocrine Neoplasia Type 2a: an 18 year experience. N Engl J Med, 318:478–484, 1988.
Tischler AS. The adrenal medulla and extra-adrenal paraganglia. In: Kovacs, K, Asa S, eds. Functional Endocrine Pathology, 2nd Ed. Blackwell Science, 1998, pp. 550–595.
Tischler AS, Lee YC, Perlman RL, Costopoulos D, Slayton VW, Bloom SR. Production of “ectopic” vasoactive intestinal peptide-like and neurotensin-like immunoreactivity in human pheochromocytoma cell cultures. J Neurosci 4:1398–1404, 1984.
Tischler AS, DeLellis RA, Biales B, Nunnemacher G, Carabba V, Wolfe HJ. Nerve growth factor-induced neurite outgrowth from normal human chromaffin cells. Lab Invest 43:398–409, 1980.
Grakner HG. Monoclonal antibody, to 5-bromo- and 5-iodo-deoxyuridine: A new agent for detection of DNA replication. Science 218:474–475, 1982.
Tischler AS, Ruzicka LA, Riseberg J. Immunocytochemical analysis of chromaffin cell proliferationin vitro. J Histochem Cytochem 40:1043–1045, 1992.
Tischler AS, Riseberg J. Different responses to mitogenic agents by adult rat and human chromaffin cellsin vitro. Endocr Pathol 4:15–19, 1993.
Johnson CM, Hill CS, Chawla S, Treisman R, Bading H. Calcium controls gene expression via three distinct pathways that can function independently of theras/mitogen-activated protein kinase (ERKs) signalling cascade. J Neurosci 17:6189–6202, 1997.
Kotzbauer PT, Lampe PA, Heukeroth RO, Golden JP, Creedon DJ, Johnson EM Jr, et al. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature 384:467–470, 1996.
Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, et al. A GPI-linked protein that interacts with ret to form a candidate neurturin receptor. Nature 387:717–721, 1997.
Bennett D, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 18:3059–3072, 1998.
Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kineses are activated by collagen. Mol Cell 1:13–23, 1997.
Myers MP, Swanson KD, Landreth G. Mechanisms of growth-factor mediated signal transduction in PC12 cells. In: Nelkin BD, ed. Genetic Mechanisms in Multiple Endocrine Neoplasia Type 2. Landes, Austin, TX, 1996; 62–98.
Mark MD, Storm DR. Antiproliferative activity of KCI contributes to EGF-induced neurite outgrowth in PC12 cells. Neurosci Lett 230:73–76, 1997.
Greenwald I, Rubin GM. Making a difference. The role of cell-cell interactions in establishing separate identities for equivalent cells. Cell 68:271–281, 1992.
Marshall CJ. Specificity of receptor tyrosine kinase signalling: Transient versus sustained extracellular signal-regulated activation. Cell 80:1079–1090, 1995.
Chen H, Hall S, Heffernan B, Thompson NT, Rogers MV, Rhodes J. Convergence of Schiff base costimulatory signalling and TCR signalling at the level of mitogen-activated protein kinase ERK2. J Immunol 159:2274–2281, 1997.
Veeranna, Amin N, Ahn N, Jaffe H, Winters C, Grant P, Pant H. Mitogen-Activated Protein Kinases (ERK1,2) Phosphorylate Lys-Ser-Pro (KSP) Repeats in Neurofilament Proteins NF-H and NF-M. J Neurosci 18:4008–4021, 1998.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Powers, J.F., Tsokas, P. & Tischler, A.S. Theret-activating ligand GDNF is differentiative and not mitogenic for normal and neoplastic human chromaffin cells in vitro. Endocr Pathol 9, 325–331 (1998). https://doi.org/10.1007/BF02739692
Issue Date:
DOI: https://doi.org/10.1007/BF02739692