Summary
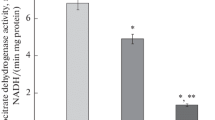
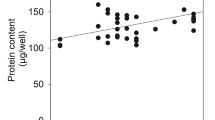
This study examines the factors involved in the rapid glycolysis and glycogenolysis that occur during the first stages of hepatocyte culture: a) Shortly after seeding glycolysis, estimated as lactate released to culture medium, increased 10 times in comparison to that reported in vivo. By 8 to 9 h of culture, hepatocytes were nearly glycogen-depleted even in the presence of insulin. b) 6-Phosphofructo-2-kinase remained 100% active during this period. The proportion of the initial active phosphorylase (87%) decreased to 57% by 7 h of culture. c) Fructose 2,6-bisphosphate content was initially similar to that found in liver of fed animals, decreased after seeding and increased thereafter up to four times the initial concentration. In spite of changes in the concentration of this activator, the glycolytic rate remained high and constant. d) ADP and AMP increased sharply after cell plating, reaching values 1.7 and 3.5 times higher. The rise in AMP levels may be involved in the activation of glycolysis and glycogenolysis, because this metabolite is known to act as an allosteric activator of phosphofrucktokinase and glycogen phosphorylase. This metabolic situation resembles that of cells under hypoxia.
Similar content being viewed by others
References
Adam, H. Determination of adenosine-5′-diphosphate and adenosine-5′-monophosphate. In: Bergmeyer, H. U., ed. Methods in enzymatic analysis. New York: Academic Press; 1965:253–259.
Bartrons, R.; Hue, L.; Van Schaftingen, E., et al. Hormonal control of fructose 2,6 bisphosphate concentration in isolated rat hepatocytes. Biochem. J. 214:829–837; 1983.
Bernaert, D.; Wanson, J. C.; Drochmens, P., et al. Effect of insulin on ultrastructure and glycogenesis in primary cultures of adult rat hepatocytes. J. Cell. Biol. 74:878–900; 1977.
Berry, M. N.; Friend, D. S. High yield preparation of isolated rat liver parenchymal cell. J. Cell Biol. 43:506–520; 1969.
Bissell, M.; Hammaker, L. E.; Meyer, U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture: I. Functional studies. J. Cell. Biol. 59:722–734; 1973.
Blaauboer, B. J.; Paine, A. J. Attachment of rat hepatocytes to plastic substrata in the absence of serum requires protein synthesis. Biochem. Biophys. Res. Com. 90:368–374; 1979.
Bollen, M.; De Ruysscher, D.; Stalmans, W. On the mechanism of hepatic glycogenolysis induced by anoxia or cyanide. Biochem. Biophys. Res. Com. 115:1033–1039; 1983.
Castell, J. V.; Gomez-Lechon, M. J.; Coloma, J., et al. Preservation of the adult functionality of hepatocytes in serumfree cultures. In: Fischer, G.; Wieser, R. J., eds. Hormonally defined media: a tool in cell biology. Berlin: Springer-Verlag; 1983:333–336.
Edwards, K.; Urban, J.; Schreiber, G. Relationships between protein synthesis and secretion in liver cells and the state of adenine nucleotide system. Aust J. Biol. Sci. 32:299–307; 1979.
El-Maghrabi, M. N.; Pilkis, S. J. Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a review of relationships between the two activities of the enzyme. J. Cell. Biochem. 26:1–17; 1984.
Furuya, E.; Uyeda, K. An activation factor of liver phosphofructokinase. Proc. Natl. Acad. Sci. USA 77:5861–5864; 1980.
Gomez-Lechon, M. J.; Castell, J. V. The role of fetal calf serum during the first stages of hepatocyte culture. In: Fischer, G.; Wieser, R. J., eds. Homonally defined media: a tool in cell biology. Berlin: Springer-Verlag; 1983:340–343.
Gomez-Lechon, M. J.; Lopez, M. P.; Castell, J. V. Biochemical functionality and recovery of hepatocytes after deep-freezing storage. In Vitro 20:826–832; 1984.
Guguen-Guillouzo, C.; Guillouzo, A. Modulation of functional activities in cultured rat hepatocytes. Mol. Cell. Biochem. 53/54:35–56; 1983.
Gutmann, I.; Wahlefeld, A. W. Determination ofl-(+)-lactate with lactate dehydrogenase and NAD. In: Bergmeyer, H. U., ed. Methods of enzymatic analysis, vol. 4. New York: Academic Press; 1974:1464–1468.
Hassid, W. Z.; Abraham, S. Chemical procedures for analysis of polysaccharides. In: Colowick, S. P.; Kaplan, N. O., eds. Methods in Enzymology, vol. 3. New York: Academic Press; 1957:37.
Hems, D. A.; Whitton, P. D. Control of hepatic glycogenolysis. Physiol. Rev. 60:1–50; 1980.
Hers, H. G.; Hue, L. Gluconeogenesis and related aspects of glycolysis. Ann. Rev. Biochem. 52:617–653; 1983.
Hers, H. G.; Van Schaftingen, E. Fructose 2,6 bisphosphate 2 years after its discovery. Biochem. J. 206:1–12; 1982.
Holzer, C.; Meier, P. Maintenance of periportal and pericentral oxygen tensions in primary rat hepatocyte cultures: influence on cellular DNA and protein content monitored by flow cytometry. J. Cell. Physiol. 133:297–303; 1987.
Horiuti, Y.; Nakamura, T.; Ichihara, A. Role of serum in the maintenance of functional hepatocytes in primary culture. J. Biochem. 92:1985–1994; 1982.
Hue, L. Role of fructose 2,6 bisphosphate in the stimulation of glycolysis by anoxia in isolated hepatocytes. Biochem. J. 206:359–365; 1982.
Hue, E.; Blackmore, P. F.; Shikana, H., et al. Regulation of fructose 2,6 bisphosphate content in rat hepatocytes, perfused hearts and perfused hindlimbs. J. Biol. Chem. 257:4308–4313; 1982.
Ichihara, A.; Nakamura, T.; Tanaka, K., et al. Biochemical functions of adult rat hepatocytes in primary culture. Ann. N.Y. Acad. Sci. 349:77–86; 1980.
Jauregui, H. O.; McMillan, P. N.; Driscoll, J., et al. Attachment and long-term survival of adult rat hepatocytes in primary monolayer cultures: comparison of different substrata and tissue culture media formations. In Vitro 22:13–22; 1986.
Jeejeebhoy, K. N.; Phillips, M. J.; Ho, J., et al. Ultrastructural and functional studies of cultured adult rat hepatocytes. A. Ann. N.Y. Acad. Sci. 349:18–27; 1980.
Katz, J.; McGarry, J. D. The glucose paradox. Is glucose a substrate for liver metabolism?. J. Clin. Invest. 74:1901–1909; 1984.
Katz, J.; Wals, P. A.; Golden, S., et al. Recycling of glucose by rat hepatocytes. Eur. J. Biochem. 60:91–101; 1975.
Laishes, B. A.; Williams, G. M. Conditions affecting primary cells cultures of functional adult rat hepatocytes: 1. Effect of insulin. In Vitro 12:521–532; 1976.
Lampretch, N.; Trautschold I. ATP determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer, H. U., ed. Methods of enzymatic analysis. New York: Academic Press; 1965:543–551.
López, M. P.; Gómez-Lechón, M. J.; Castell, J. V. Glycogen synthesis in serum free cultured hepatocytes in response to insulin and dexamethasone. In Vitro 20:923–931; 1984.
Lowry, O. H.; Rosebrough, N. J.; Farr, A. L., et al. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275; 1951.
Lutaya, G.; Sharma, R. J.; Griffiths, J. R. Glycogenolysis in liver of phosphorylase kinase-deficient rats during liver perfusion and ischaemia. Biochem. J. 214:645–648; 1983.
Neely, P.; El-Maghrabi, M. R.; Pilkis, S. J. et al. Effect of diabetes, insulin, starvation and refeeding on the level of rat hepatic fructose 2,6 bisphosphate. Diabetes 30:1062–1064; 1981.
Pilkis, S. J.; Chrisam, J. D.; El-Maghrabi, M. R., et al. The action of insulin on hepatic fructose 2,6 bisphosphate metabolism. J. Biol. Chem. 258:1495–1503; 1983.
Probst, I.; Jungermann, K. Short-term regulation of glycolysis by insulin and dexamethasone in cultured rat hepatocytes. Eur. J. Biochem. 135:151–155; 1983.
Probst, I.; Schwartz, P.; Jungermann, K. Induction in primary culture of gluconeogenic and glycolytic hepatocytes resembling periportal and perivenous cells. Eur. J. Biochem. 126:271–278; 1982.
Probst, I.; Unthan-Fechner, k. Activation of glycolysis by insulin with a sequential increase of the 6-phosphofructo-2-kinase activity, fructose 2,6 bisphosphate level and pyruvate kinase activity in cultured rat hepatocytes. Eur. J. Biochem. 153:347–353; 1985.
Richards, C. S.; Furuya, E.; Uyeda, K. Regulation of fructose 2,6 bisphosphate concentration in isolated hepatocytes. Biochem. Biophys. Res. Com. 100:1673–1679; 1981.
Schudt, C. Regulation of glycogen synthesis in rat hepatocyte cultures by glucose, insulin and glucocorticoids. Eur. J. Biochem. 97:155–160; 1979.
Schudt, C. Influence of insulin, glucocorticoids and glucose on glycogen synthase activity in hepatocyte cultures. Biochim. Biophys. Acta 629: 499–509; 1980.
Schwarze, P. E.; Solheim, A. E.; Seglen, P. O. Aminoacid and energy requirements for rat hepatocyte in primary culture. In Vitro 18:43–54; 1982.
Sharma, R. J.; Rodrigues, L. M.; Whitton, P. D., et al. Control mechanism in the acceleration of hepatic glycogen degradation during hypoxia. Biochim. Biophys. Acta 630:414–424; 1980.
Stalmans, W.; Hers, H. G. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate; a technical note on the specific determination of the a and b forms of liver glycogen phosphorylase. Eur. J. Biochem. 54:341–350; 1975.
Trowell, D. A. Isolated liver perfusion in the study of hepatic function. J. Physiol. 100:432–435; 1942.
Van Sohaftingen, E.; Hers, H. G. Phosphofructokinase 2, the enzyme that forms fructose 2,6 bisphosphate from fructose-6-phosphate and ATP. Biochem. Biophys. Res. Com. 101:1078–1084; 1981.
Van Schaftingen, E.; Jett, M. F.; Hue, L., et al. Control of liver 6-phosphofructokinase by fructose 2,6 bisphosphate and other effectors. Proc. Natl. Acad. Sci. USA 78:3483–3486; 1981.
Van Schaftingen, E.; Lederer, B.; Bartrons, R., et al. A kinetic study of pyrophosphate:fructose-6-phosphate phosphotransferase from potato tubers. Eur. J. Biochem. 129:191–195; 1982.
Williams, G. M.; Bermudez, E.; Scaramuzziono, D. Rat hepatocyte primary cell cultures. III. Improved dissociation and attachment techniques and the enhancement of survival by culture medium. In Vitro 13:809–816; 1977.
Williamson, D. H.; Brosnan, J. T. Concentrations of metabolites in animal tissues. In: Bergmeyer, H. U., ed. Methods of enzymatic analysis, vol. 4. New York: Academic Press; 1974:2266–2302.
Wirthensohn, K.; Barth, C. A. Influence of hormones and growth factors on viability, DNA and protein content of adult hepatocytes in primary culture. In Vitro 21:346–351; 1985.
Author information
Authors and Affiliations
Additional information
Part of this work was presented at the 38th Annual Meeting of the Tissue Culture Association, Washington, DC, May 1987.
Rights and permissions
About this article
Cite this article
López, M.P., Gómez-Lechón, M.J. & Castell, J.V. Active glycolysis and glycogenolysis in early stages of primary cultured hepatocytes. Role of AMP and fructose 2.6-bisphosphate. In Vitro Cell Dev Biol 24, 511–517 (1988). https://doi.org/10.1007/BF02629084
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02629084