Abstract
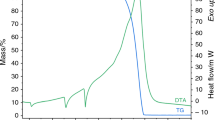
Non-isothermal dehydration of copper chloride dihydrate and nickel chloride hexahydrate were studied by using TG, DTG, DTA and DSC measurements. The copper chloride salt loses its two water molecules in one step while nickel chloride salt dehydrates in three consecutive steps. The first two steps involve the loss of 4 water molecules in two overlapped steps while the third step involves the dehydration of the dihydrate salt to give the anhydrous NiCl2.
Activation energies (ΔE) and the frequency factor (A) were calculated from DTG and DTA results. We have also calculated the different thermodynamic parameters, e.g. enthalpy change (ΔH), heat capacity (C p) and the entropy change (ΔS) from DSC measurements for both reactants.
The isothermal rehydration of the completely dehydrated salts was studied in air and under saturated vapour pressure of water. Anhydrous nickel chloride was found to rehydrate in three consecutive steps while the copper salt rehydrated in one step.
Zusammenfassung
Mittels TG-, DTG-, DTA- und DSC-Messungen wurde die nichtisothermische Dehydratation von Kupferchlorid-Dihydrat und Nickelchlorid-Hexahydrat untersucht. Das Kupferchloridsalz setzt seine zwei Moleküle Wasser in einem Schritt frei, das Nickelchloridsalz dagegen in drei aufeinanderfolgenden Schritten. Davon umfassen die ersten zwei Schritte die Freisetzung von 4 Molekülen Wasser in zwei überlappenden Schritten, während der dritte Schritt vom Dihydratsalz zur Formung von wasserfreiem NiCl2 führt.
Die Aktivierungsenergien (ΔE) und der Frequenzfaktor (A) wurden anhand von DTG- und DTA-Ergebnissen berechnet. Weiterhin berechneten wir anhand von DSC-Messungen auch thermodynamische Parameter, wie z.B. die Enthalpiedifferenz (ΔH), die Wärmekapazität (C p ) und die Entropiedifferenz (ΔS) für beide Reaktanden.
In Luft und bei Sättigungsdampfdruck für Wasser wurde die isotherme Rehydratation der vollständig dehydratierten Salze untersucht. Wasserfreies Nickelchlorid rehydratiert in drei aufeinanderfolgenden Schritten, das Kupfersalz dagegen rehydratiert nur in einem Schritt.
Similar content being viewed by others
References
M. E. Brown, D. Dollimore and A. K. Galwey, Comprehensive Chemical Kinetics, (Ed. C. H. Bamford and C. F. H. Tipper), Elsevier, Amsterdam 1980, Vol. 22.
M. E. Brown, H. Kelly, A. K. Galwey and M. A. Mohamed, Thermochim. Acta, 127 (1988) 139.
A. K. Galwey, M. A. Mohamed, S. Rajam and M. E. Brown, J. Chem. Soc. Faraday Trans. I, 84 (1988) 1349.
R. E. Grim, Clay Mineralogy, 2nd ed., McGraw-Hill, New York 1968.
D. Dollimore, Selected Annual Reviews of the Analytical Sciences, 2, 1 (L. S. Bark, Ed.), 1973 (Soc. Anal. Chem.).
D. Dollimore and D. L. Griffiths, J. Thermal Anal., 2 (1970) 229.
S. Bretsznaider and Z. Roikowski, Bull. Acad. Pol. Sci. Chim., 17 (1969) 139.
A. K. Galwey and M. A. Mohamed, Thermochim. Acta, 121 (1987) 97.
A. K. Galwey, R. Spinicci and G. G. T. Guarini, Proc. R. Soc. London, Ser. A, 378 (1981) 477.
A. K. Galwey, R. Reed and G. G. T. Guarini, Nature (London), 283 (1980) 52.
R. K. Osterheld and P. R. Bloom, J. Phys. Chem., 82 (1978) 1591.
D. Petzold and T. Naumann, J. Thermal Anal., 20 (1981) 71.
A. K. Galwey and G. M. Laverty, Thermochim. Acta, 138 (1989) 115.
M. A. Mohamed and S. A. Halawy, Bull. Fac. Sci., Assiut Univ., 19(2-B), (1990) 107.
R. C. Weast (Ed.), Handbook of Chemistry and Physics, (62nd ed.) CRC Press, New York 1982.
M. A. Mohamed, S. A. A. Mansour and M. I. Zaki, Thermochim. Acta 138 (1989) 309.
J. W. Dodd and K. H. Tonge, Thermal Methods, Analytical Chemistry by Open Learning, J. Wiley, New York 1987.
C. Heald and A. C. K. Smith, Applied Physical Chemistry, Macmillan Press Ltd., London 1982, pp. 20–40.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohamed, M.A., Halawy, S.A. Non-isothermal kinetic and thermodynamic study for the dehydration of copper(II) chloride dihydrate and nickel chloride hexahydrate. Journal of Thermal Analysis 41, 147–159 (1994). https://doi.org/10.1007/BF02547020
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02547020