Abstract
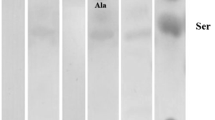
Hippocampal cholinergic neurostimulating peptide (HCNP) stimulates cholinergic activity of cultured medial septal nuclei explants. It consists of eleven amino acids that are located at the N-terminal region of its precursor protein. This report concerns the demonstration and characterization of an HCNP processing enzyme that cleaves the bioactive undecapeptide from the precursor. The enzyme was purified from the hippocampus of young Wistar rats. A synthetic deacetylated peptide (peptide1–26) consisting of the N-terminal 26 amino acids of the HCNP precursor protein served as substrate. The product of the enzyme reaction was identified and quantitated by HPLC using deacetylated HCNP as standard. The amount of undecapeptide generated was directly proportional to the time of incubation of the enzyme reaction mixture. From molecular sieving chromatography it was estimated that the molecular mass of the enzyme is close to 68 kDa. The HCNP processing enzyme has a pH optimum of 6.0 and a Km of 0.50 mM for peptide1–26. Preincubation at 56°C causes rapid inactivation of the HCNP processing activity. Enzyme activity is enhanced by EDTA and 1,4-dithiothreitol, and inhibited by antipain, chymostatin and E-64. These findings suggest that the enzyme probably has a thiol group in its active site. This novel enzyme of the hippocampus may represent a valuable tool for further studies on the general protein metabolism in the central nervous system, as well as for elucidating the neurochemical aspects of neurodegenerative disorders.
Similar content being viewed by others
References
Loh, Y. P., Brownstein, M. J., and Gainer, H. 1984. Proteolysis in neuropeptide processing and other neural functions. Ann. Rev. Neurosci. 7:189–222.
Gainer, H., Russell, J. T., and Loh, Y. P. 1985. The enzymology and intracellular organization of peptide precursor processing: the secretory vesicule hypothesis. Neuroendocrinology 40:171–184.
Docherty, K., and Steiner, D. F. 1982. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu. Rev. Physiol. 44:625–638.
Andrews, P. C., Brayton, K., and Dixon, J. E. 1987. Precursors to regulatory peptides: their proteolytic processing. Experientia 43:784–790.
Steiner, D. F., Smeekens, S. P., Ohagi, S., and Chan, S. J. 1992. The new enzymology of precursor processing endoproteases, J. Biol. Chem. 267:23435–23438.
Fuller, R. S., Brake, A. J., and Thorner, J. 1989. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science 246:482–486.
Seidah, N. G., Gaspar, L., Mion, P., Marcinkiewicz, M., Mbikay, M., and Chrétien, M. 1990. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candiadates for pro-hormone processing proteinases. DNA Cell Biol. 9:415–424.
Smeekens, S. P., and Steiner, D. F. 1990. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J. Biol. Chem. 265:2997–3000.
Smeekens, S. P., Avruch, A. S., LaMendola, J., Chan, S. J., and Steiner, D. F. 1991. Identification of cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc. Natl. Acad. Sci. USA 88:340–344.
Loh, Y. P., Parish, D. C., and Tuteja, R. 1985. Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J. Biol. Chem. 260:7194–7205.
Krieger, T. J., and Hook, V. Y. H. 1991. Purification and characterization of a novel thiol protease involved in processing the enkephalin precursor. J. Biol. Chem. 266:8376–8383.
Bourdais, J., Pierotti, A. R., Boussetta, H., Barre, N., Devilliers, G., and Cohen, P. 1991. Isolation and functional properties of an arginine-selective endoprotease from rat intestinal mucosa. J. Biol. Chem. 266:23386–23391.
Viereck, J. C., and Beinfeld, M. C. 1992. Characterization of cholecystokinin 8-generating endoprotease purified from rat brain synaptosomes. J. Biol. Chem. 267:19475–19481.
Hudson, P., Haley, J., Cronk, M., Shine, J., and Niall, H. 1981. Molecular cloning and characterization of cDNA sequences coding for rat relaxin, Nature 291:127–131.
Tager, H. S., Emdin, S. O., Clark, J. L., and Steiner, D. F. 1973. Studies on the conversion of proinsulin to insulin. J. Biol. Chem. 248:3476–3482.
Jörnvall, H., Carlquist, M., Kwauk, S., Otte, S. C., McIntosh, C. H. S., Brown, J. C., and Mutt, V. 1981. Amino acid sequence and heterogeneity of gastric inhibitory polypeptide (GIP). FEBS Lett. 123:205–210.
Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K., and Masaki, T. 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415.
Ojika, K., and Appel, S. H. 1984. Neurotrophic effects of hippocampal extracts on medial septal nucleus in vitro. Proc. Natl. Acad. Sci. USA 81:2567–2571.
Ojika, K., Kojima, S., Ueki, Y., Fukushima, N., Hayashi, K., and Yamamoto, M. 1992. Purification and structural analysis of hippocampal cholinergic neurostimulating peptide. Brain Res. 572:164–171.
Ojika, K., Mitake, S., Kamiya, T., Kosuge, N., and Taiji, M. 1994. Two different molecules, NGF and free-HCNP, stimulate cholinergic activity in septal nuclei in vitro in different manner. Dev. Brain Res. 79:1–9.
Tohdoh, N., Tojo, S., Agui, H., and Ojika, K. 1995. Sequence homology of rat and human HCNP precursor protein, bovine phosphatidylethanolamine-binding protein and rat 23-kDa protein associated with the opioid-binding protein. Mol. Brain Res. 30:381–384.
Grandy, D. K., Hanneman, E., Bunzow, J., Shih, M., Machida, C. A., Bidlack, J. M., and Civelli, O. 1990. Purifiction, cloning, and tissue distribution of a 23-kDa rat protein isolated by morphine affinity chromatography. Mol. Endocrinol. 4:1370–1376.
Schoentgen, F., Saccoccio, F., Jollès, J., Bernier, I., and Jollès, P. 1987. Complete amino acid sequence of a basic 21-kDa protein from bovine brain cytosol. Eur. J. Biochem. 166:333–338.
Ojika, K., Katada, E., Matsukawa, N., Tsugu, Y., Otsuka, Y., Mitake, S., and Tohdoh, N. 1995. Demonstration of deacetylated hippocampal cholinergic neurostimulating peptide (HCNP) and HCNP precursor protein in rat tissues. Brain Res. 701:19–27.
Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72:248–254.
Loh, Y. P., Tam, W. W. H., and Russell, J. T. 1984. Measurement of ΔpH and membrane potential in secretory vesicles isolated from bovine pituitary intermediate lobe. J. Biol. Chem. 259:8238–8245.
Mitake, S., Suzuki, T., Okumura-Noji, K., Katada, E. and Ojika, K. 1994. Presynaptic localization of hippocampal cholinergic neurostimulating peptide (HCNP) immunoreactivity. Society for Neuroscience 20:517 (abstract).
Suda, H., Aoyagi, T., Hamada, M., Takeuchi, T., and Umezawa, H. 1972. Antipain, a new protease inhibitor isolated from Actinomycetes. J. Antibiotics 25:263–266.
Hanada, K., Tamai, M., Yamagishi, M., Ohmura, S. Sawada, J., and Tanaka, I. 1978. Isolation and characterization of E-64, a new thiol protease inhibitor. Agric. Biol. Chem. 42:523–528.
Murachi, T. 1983. Calpain and calpastatin. Trends Biochem. Sci. 8:167–169.
Towatari, T., Kawabata, Y., and Katunuma, N. 1979. Crystallization and properties of cathepsin B from rat liver. Eur. J. Biochem. 102:279–289.
Schwartz, W. N., and Barrett, A. J. 1980. Human cathepsin H. Biochem. J. 191:487–497.
Kirschke, H., Langner, J., Wiederanders, B., Ansorge, S., and Bohley, P. 1977. Cathepsin L. Eur. J. Biochem. 74:293–301.
Folk, J. E., and Cole, P. W. 1965. Chymotrypsin C. J. Biol. Chem. 240:193–197.
Umezawa, H., Aoyagi, T., Morishima, H., Kunimoto, S., Matsuzaki, M., Hamada, M., and Takeuchi, T. 1970. Chymostatin, a new chymotrypsin inhibitor produced by Actinomycetes. J. Antibiotics 23:425–427.
Selkoe, D. J., Bell, D. S., Podlisny, M. B., Price, D. L., and Cork, L. C. 1987. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science 235:873–877.
Abraham, c. R., Selkoe, D. J., and Potter, H. 1988. Immunochemical identification of the serine protease inhibitor α 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell 52:487–501.
Cataldo, A. M., Thayer, C. Y., Bird, E. D., Wheelock, T. R., and Nixon, R. A. 1990. Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer's disease: evidence for a neuronal origin. Brain Res. 513:181–192.
Ivy, G. O., Schottler, F., Wenzel, J., Baudry, M., and Lynch, G. 1984. Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science 226:985–987.
Ojika, K. and Appel, S. H. 1983. Neurotrophic factors and Alzheimer's disease. In, ed., R. Katzman, Banbury report 15: Biological Aspects of Alzheimer's disease. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY: 285–295.
Mitake, S., Ojika, K., Katada, E., Otsuka, Y., Matsukawa, N. and Fujimori, O. 1995. Distribution of hippocampal cholinergic neurostimulating peptide (HCNP) immunoreactivity in the central nervous system of the rat. Brain Res. (in press).
Mitake, S., Ojika, K., Katada, E., Otsuka, Y., Matsukawa, N., and Fujimori, O. 1995. Accumulation of hippocampal cholinergic neurostimulating peptide (HCNP)-related components in Hirano bodies. Neuropathol. Appl. Neurobiol. 21:35–40.
Hirano, A. 1994. Hirano bodies and related neuronal inclusions. Neuropathol. Appl. Neurobiol. 20:3–11.
Whitehouse, P. J., Price, D. L., Struble, R. G., Clark, A. W. Coyle, J. T., and DeLong, M. R. 1982. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239.
Goedert, M., Fine, A., Hunt, S. P., and Ullrich, A. 1986. Nerve growth factor mRNA in peripheral and central rat tissue and in the human central nervous system: lesion effects in the rat brain and levels in Alzheimer's disease. Mol. Brain Res. 1:85–92.
Goedert, M., Fine, A., Dawbarn, D., Wilcock, G. K., and Chao, M. V. 1989. Nerve growth factor receptor mRNA distribution in human brain: normal levels in basal forebrain in Alzheimer's disease. Mol. Brain Res. 5:1–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Otsuka, Y., Ojika, K. Demonstration and characterization of hippocampal cholinergic neurostimulating peptide (HCNP) processing enzyme activity in rat hippocampus. Neurochem Res 21, 369–376 (1996). https://doi.org/10.1007/BF02531654
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02531654