Abstract
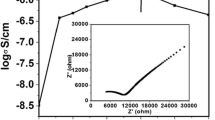
Polymeric electrolytes are very useful for their technological applications in different electrochemical devices such as batteries, electrochromic devices, smart windows, etc. One of the most studied solid electrolyte system is PEO (poly-ethylene oxide) complexed with various lithium salts. A limitation of this polymer electrolyte is low ionic conductivity. However, nanoscale manipulation of the solid polymer electrolyte has the potential to address this issue. This work discusses how it is possible to increase the PEO conductivity when this polymer is contained in nanostructures, specifically nanopores. The nanostructures used are alumina filtration membranes (thickness=6 µm, diameter=13 mm) with three different pore sizes 0.02 µm, 0.1 µm and 0.2 µm. Electrochemical characterization has been performed with an HP4194A Impedance/Gain phase analyser and Solartron 1260 Impedance/Gain phase analyser. The former instrument tests these films at a high frequency (from 100 Hz to 40 MHz) while the later at low frequency (from 1 Hz to 1 MHz). From these experiments, it has been determined that two regions of ion conduction exit. One is conduction through the bulk polymer electrolyte in the pores while the other is an interfacial conduction at the interface between the pore walls and the PEO electrolyte. The conductivity of the PEO is increased when confined in these nanostructures.
Similar content being viewed by others
References
K.H. Heckner and A. Kraft, Solid State Ionics152–153, 899 (2002).
P.G. Bruce, Elctrochimica Acta40, 2077 (1995).
M. Le Granvalet-Mancini, L. Honeycutt and D. Teeters, Elctrochimica Acta45, 1491 (2000).
M. Le Granvalet-Mancini, T. Hanrath. and D. Teeters, Solid state Ionics135, 283 (2000).
J. Kim, M.K. Park, J.Y. Bae and J.H. Ahn, Electrochemistry Communicatios3, 643 (2001).
R.N. Mason, M. Smith, T. Andrews and D. Teeters, Solid State Ionics118, 129 (1999).
I. Nicotera, G.A. Ranieri, M. Terenzi, A.V. Chadwick and M.I. Webmaster, Solid State Ionics146, 143 (2002).
E. Cazzanelli, G. Mariotto, G.B. Appetecchi, F. Croce and B. Scrosati, Elctrochimica Acta40, 2379 (1995).
I. Nicotera, C. Olivierio, G.A. Ranieri, A. Spadafora, M. Castriota and E. Cazzanelli, J. Chem. Phys.117, 7373 (2002).
I. Nicotera, L. Coppola, C. Oliviero, A. Russo and G.A. Ranieri, Solid State Ionics167, 213 (2004).
Y. Ward and Y. Mi, Polymer40, 2465 (1999).
H. Sasaki, P.K. Bala, H. Yoshida and E. Ito, Polymer36, 4805 (1995).
E. Cazzanelli, F. Croce, G.B. Appetecchi, F. Benevelli and P. Mustarelli, J. Chem. Phys.107, 5740 (1997).
M. Castriota, E. Cazzanelli, I. Nicotera, L. Coppola, C. Oliverio and G.A. Ranieri, J. Chem. Phys.118, 5537 (2003).
J.H. Shin, W.A. Henderson and S. Passerini, Electrochemical and Solid-State Letters8, A125 (2005).
M. Castriota, T. Caruso, R.G. Agostino, E. Cazzanelli, W.A. Henderson and S. Passerini, J. Phys. Chem. A109, 92 (2005).
F. Croce, G.B. Appetecchi, L. Persi and B. Scrosati, Nature394, 456 (1998).
F. Croce, B. Scrosati and G. Mariotto, Chem. Mater.4, 1134 (1992).
Z. Wen, T. Itoh, M. Ikeda, N. Hirata, M. Kubo and O. Yamamoto, J. Power Sources90, 20 (2000).
S. Vorrey and D. Teeters, Electrochimica Acta48, 2137 (2003).
A.R. Layson and D. Teeters, Solid State Ionics175, 773 (2004).
Y. Fang and J. Leddy, J. Phys. Chem.99, 553 (1995).
H. Masuda, K. Nishio and N. Baba, Thin Solids Films223, 1 (1993).
H. Masuda and K. Fukuda, Science268, 1466 (1995).
A.-P. Li, F. Muller, A. Birner, K. Neilsch, and U. Gosele, Adv. Mater.11, 483 (1999).
Author information
Authors and Affiliations
Additional information
Invited Scholar Research from: LiCryl — INFM (Liquid Crystal Regional Laboratory) c/o Department of Physics, University of Calabria, Via P. Bucci Cubo 31C, I-87036 Rende (CS) Italy
Rights and permissions
About this article
Cite this article
Castriota, M., Teeters, D. Impedance spectroscopy of PEO-lithium triflate confined in nanopores of alumina membranes. Ionics 11, 220–225 (2005). https://doi.org/10.1007/BF02430380
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02430380