Summary
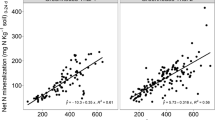
A practical fibreglass cylinder-plastic bag system has been designed for making acetylene reduction assays in the field. Thein situ assay was used to determine seasonal patterns of nitrogenase activity for the perennial forage legumesGalega orientalis, Trifolium pratense andMedicago sativa grown under stadard management in southern Filand (60° north). Nitrogenase activity was still detected in the field plots in November, when soil temperature was 1.5°C and air temperature 0.5°C. The acetylene reduction data from weekly measurements were analyzed for correlation with plant growth rate and short-term fluctuations of environmental factors. Generally, there was a good correlation between nitrogenase activity and plant growth rate. Residual fluctuations in activity were only correlated with environmental factors in one case. The nitrogenase activity ofM. sativa was dependent on air temperature in addition to growth rate. Thus, the nitrogen fixing systems in these forage legumes seem to be an integrated part of the plants, being fairly insensitive to short-term environmental changes.
Similar content being viewed by others
References
Balandreau J and Ducerf P 1978 Activité nitrogénasique (C2H2)in situ: Mesure analyse des facteurs limitants, comparison de systémes fixateurs d'azote.In Isotopes in Biological Dinitrogen Fixation. IAEA Vienna, 173–190.
Balandreau J and Ducerf P 1980 Analysis of factors limiting nitrogenase (C2H2) activity in the field.In Nitrogen Fixation vol. II. Eds. W E Newton and W H Orme-Johnson. University Press, Baltimore, pp 229–242.
Bergersen F J 1970 The quantitative relationship betwen nitrogen fixation and the acetylene reduction assay. Aust. J. Biol. Sci. 23, 1015–1025.
Van Berkum P and Day J M 1980 Nitrogenase activity associated with soil cores of grasses in Brazil. Soil. Biol. Biochem. 12, 137–140.
Berry J and Björkman O 1980 Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543.
Bethlenfalvay G J, Abu-Shakra S S and Phillips D A 1978 Interdependence of nitrogen nutrition and photosynthesis inPisum sativum L. I Effect of combined nitrogen on symbiotic nitrogen fixation and photosynthesis. Plant Physiol. 62, 127–130.
Bethlenfalvay G J, Abu-Shakra S S and Phillips D A 1978 Interdependence of nitrogen nutrition and photosynthesis inPisum sativum L. II Host plant response to nitrogen fixation by Rhizobium strains. Plant Physiol. 62, 131–133.
Dart P J and Day J M 1971 Effects of incubation temperature and oxygen tension on nitrogenase activity of legume root nodules. Plant and Soil Spec. Vol. 167–184.
Gibson A H 1976 Recovery and compensation by nodulated legumes to environmental stress.In Symbiotic Nitrogen Fixation in Plants Ed. P S Nutman. Cambridge University Press, London, pp 385–403.
Halliday J and Pate J S 1976 The acetylene reduction assay as a means of studying nitrogen fixation in white clover under sward and laboratory conditions. J. British Grassl. Soc. 31, 29–35.
Hardy R W F and Havelka U D 1976 Photosynthate as a major factor limiting nitrogen fixation by field-grown legumes with emphasis on soybeans.In Symbiotic Nitrogen Fixation in Plants. Ed. P S Nutman Cambridge University Press, London, pp 421–439.
Hardy R W F, Holsten R D, Jackson E K and Burns R C 1968 The acetylene-ethylene assay for N2-fixation: laboratory and field evaluation. Plant Physiol. 43, 1185–1205.
Hopmans P, Douglas L A and Chalk P M 1982 Estimation of nitrogen fixation byTrifolium subterraneum L. andMedicago truncatula Gaertn. grown in pots using a nondestructive acetylene reduction assay. Soil Biol. Biochem. 14, 495–500.
Lee K K and Watanabe J 1977 Problems of the acetylene reduction technique applied to water-saturated paddy soils. Appl. Environ. Microbiol. 34, 654–660.
Lee K K and Yoshida T 1977 An assay technique of measurement of nitrogenase activity in root zone of rice for a varietal screening by the acetylene reduction method. Plant and Soil 46, 127–134.
Masterson C L and Murphy P M 1976 Application of the acetylene reduction technique to the study of nitrogen fixation by white clover in the field.In Symbiotic Nitrogen Fixation in plants. Ed. P S Nutman. Cambridge University Press, London, pp 299–316.
Masterson C L and Murphy P M 1980 The acetylene reduction technique.In Recent Advances in Biological Nitrogen Fixation. Ed. N S Subba-Rao. Edward Arnold Publishers Limited, London, pp 8–33.
Minchin F R and Pate J S 1974 Diurnal functioning of the legume root nodule. J. Exp. Bot. 25, 295–308.
Moustafa E, Ball R and Field T R D 1969 The use of acetylene reduction to study the effect of nitrogen fertilizer and defoliation on nitrogen fixation by field-grown white clover. N. Z. J. Agric. Res. 12, 691–696.
Murphy P M 1981 Effect of photoperiod on N2 (C2H2) fixation and H2 production by white clover.In Current Perspectives in Nitrogen Fixation. Eds. A H Gibson and W E Newton. Australian Academy of Science, Canberra, p. 463.
Pàte J S 1976 Physiology of the reaction of nodulated legumes to environment.In Symbiotic Nitrogen Fixation in Plants. Ed. P S Nutman. Cambridge University Press, London, pp 335–360.
Raper C D and Patterson R P 1980 Environmental sensitivity of acetylene reduction activity in prediction of nitrogen fixation in soybeans. Agron. J. 72, 717–719.
Rice W A 1980 Seasonal patterns of nitrogen fixation and dry matter production by clovers grown in the Peace River region. Can. J. Plant Sci. 60, 847–858.
Rice W A and Paul E A 1971 The acetylene reduction assay for measuring nitrogen fixation in waterlogged soil. Can. J. Microbiol. 17, 1049–1056.
Sprent J J 1976 Nitrogen fixation by legumes subjected to water and light stress. Symbiotic nitrogen fixation in plants. Ed. P S Nutman. Cambridge University Press, London, pp 405–420.
Taylor L R 1961 Aggregation, variance and the mean. Nature London 189, 732–735.
Wheeler C T and Lawrie A C 1976 Nitrogen fixation in root nodules of alder and pea in relation to the supply of photosynthetic assimilates.In Symbiotic Nitrogen Fixation in Plants. Ed. P S Nutman. Cambridge University Press, London, pp 497–503.
Witty J F and Day J M 1978 Use of15N2 in evaluating asymbiotic N2 fixation.In Isotopes in Biological Dinitrogen Fixation. IAEA Vienna, 135–149.
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Helge Gyllenberg on the occasion of his 60th birthday.
Rights and permissions
About this article
Cite this article
Lindström, K. Analysis of factors affectingin situ nitrogenase (C2H2) activity ofGalega orientalis, Trifolium pratense andMedicago sativa in temperate conditions. Plant Soil 79, 329–341 (1984). https://doi.org/10.1007/BF02184326
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02184326