Abstract
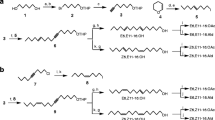
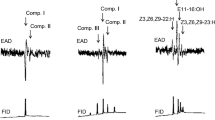
Pheromone compounds so far identified from most geometrid moths consist of all-Z diene, triene, or tetraene hydrocarbons with chain lengths of C17 to C21, and their monoepoxide derivatives biosynthesized from linoleic and linolenic acids. The present study reports the occurrence of olefinic acetates as sex pheromones in three species of Geometridae. (Z,Z)-9,11-tetradecadienyl acetate and (Z,Z)-7,9-dodecadienyl acetate found in female gland extracts ofIdaea aversata elicited significant responses from conspecific male antennae in gas chromatography with electroantennographic detection (GCEAD). In extracts ofI. straminata, (Z,Z)-7,9-dodecadienyl acetate, (E,Z)-7,9-dodecadienyl acetate, and (Z,Z)-7,9-dodecadienyl acetate were found, and the synthetic compounds elicited strong responses from conspecific male antennae. In the third species,I. biselata, only (Z,Z)-7,9-dodecadienyl acetate was found in the female extracts, and this compound elicited a strong EAD response from the conspecific male antenna. The identities of the pheromone components inI. aversata andI. straminata were further confirmed according to their characteristic ions after GC-MS analyses. Single sensillum recordings fromI. aversata showed two types of pheromone-detecting sensilla present on the male antenna. One type contained two receptor neurons, one of which was specifically tuned to (Z,Z)-9,11-tetradecadienyl acetate, the other to (Z,E)-9,11-tetradecadienyl acetate. A second type contained one neuron responding to (Z,Z)-7,9-dodecadienyl acetate. The two types were clearly different also with respect to external morphology, the former being considerably longer and having a larger base diameter. Also inI. straminata two physiological types of sensilla could be distinguished. One type contained two neurons, one of which responded to (Z,Z)-7,9-dodecadienyl acetate, the other to (Z,E)-9,11-tetradecadienyl acetate. The second type contained one neuron, responding to (Z,Z)-7,9-dodecadienyl acetate. No correlation between external morphology and physiological response of the investigated sensilla was observed inI. straminata. In field tests, a two-component blend containing (Z,Z)-9,11-tetradecadienyl acetate and (Z,Z)-7,9-dodecadienyl acetate in a ratio of 10:1 was attractive to males ofI. aversata. This two-component blend was also attractive to males ofI. straminata, but in a ratio of 1:1. High numbers of maleI. biselata were caught in traps baited with (Z,Z)-7,9-dodecadienyl acetate alone. The incorporation of deuterium labels into pheromone components after topical application of deuterium-labeled palmitic acid confirmed that the pheromone components ofI. aversata could be synthesized from this precursor, as has been previously observed for acetate pheromone components of many other moth species. Our results suggest that an evolutionary reversal back to the production of palmitic acid-derived pheromone components has occurred within the geometrid subfamily Sterrhinae.
Similar content being viewed by others
References
Ando, T., Toshida, S., Tatsuki, S., andTakahashi, N. 1977. Sex attractants for male Lepidoptera.Agric. Biol. Chem. 41:1485–1492.
Ando, T., Koike, M., Uchiyama, M., andKuroko, H. 1987. Lepidopterous sex attractants with a conjugated diene system.Agric. Biol. Chem. 51:2691–2694.
Arn, H., Töth, M., andPriesner, E. 1992. List of sex pheromones of Lepidoptera and related attractants. OILB-SROP Publ., Paris.
Biwer, G., Lalanne-Cassou, B., Descoins, C., andSamain, D. 1975. Sex trapping ofSterrha biselata (Lepidoptera: Geometridae, Sterrhinae) by 7E,9Z-dodecadienyl acetate, a sex pheromone forLobesia botrana (Lepidoptera: Tortricidae, Olethreutinae).Soc. R. Hebd. Seances Acad. Sci. Ser. D. Sci. Nat. 280:1469–1472.
Bjostad, L. B., Wolf, W. A., andRoelofs, W. L. 1987. Biology and ultrastructure of sex pheromone-producing glands, pp. 77–120,in G. D. Prestwich and G. J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, New York.
Boland, W., Schroer, N., Sieler, C., andFelgel, M. 1987. Stereospecific synthesis and spectroscopic properties of isomeric 2,4,6,8-undecatetraenes. New hydrocarbons from the marine brown algaGiffordia mitchellae.Helv. Chim. Acta 70:1025–1040.
Brown, D. F., andMcDonough, L. M. 1986. Insect sex pheromones: Formulation to increase the stability of conjugated dienes.J. Econ. Entomol. 79:922–927.
Brückner, C., Buschmann, E., Becker, R., Seufert, W., de Kramer, J. J., andKrieg, W. 1988. A new highly effective synthetic pheromone mimic forLobesia botrana (Lepidoptera: Tortricidae).Z. Naturforsch. 43c:315–318.
Den Otter, C. J. 1977. Single sensillum responses in the maleAdoxophyes orana (FvR) to female sex pheromone components and their geometrical isomers.J. Comp. Physiol. 121:205–222.
Gries, G., Gries, R., Borden, J. H., Li, J., Slessor, K. N., King, G. G. S., Bowers, W. W., West, R. J., andUnderhill, E. W. 1991. 5,11-Dimethylheptadecane and 2,5-dimethylheptadecane: Sex pheromone components of the geometrid moth,Lambdina fiscellaria fiscellaria.Naturwissenschaften 78:315–317.
Gries, G., Gries, R., Krannitz, S. H., Li, J., King, G. G. S., Slessor, K. N., Borden, J. H., Bowers, W. W., West, R. J., andUnderhill, E. W. 1993a. Sex pheromone of the western hemlock looper,Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae).J. Chem. Ecol. 19:1009–1019.
Gries, G., King, G. G. S., Gries, R., Wimalaratne, P. D. C., Gray, T. G., Shepherd, R. F., Li, J., Slessor, K. N., andKhaskin, G. 1993b. 3,13-Dimethylheptadecane: Major sex pheromone component of the western false hemlock looper,Nepytia freemani Munroe (Lepidoptera: Geometridae).J. Chem. Ecol. 19:1501–1510.
Hall, D. R., Beevor, P. S., Lester, R., Poppi, R. G., andNesbitt, B. F. 1975. Synthesis of the major sex pheromone of the Egyptian cotton leafwormSpodoptera littoralis (Boisd.).Chem. Ind. 1975:216–217.
Hansen, K. 1984. Discrimination and production of disparlure enantiomers by the gypsy and the nun moth.Physiol. Entomol. 9:9–18.
Hansson, B. S., Löfstedt, C., andRoelofs, W. L. 1987. Inheritance of olfactory response to sex pheromone components inOstrinia nubilalis.Naturwissenschaften 74:497–499.
Hansson, B. S., Szöcs, G., Schmidt, F., Francke, W., Löfstedt, C., andToth, M. 1990. Electrophysiological and chemical analysis of sex pheromone communication system of the mottled umber,Erannis defoliaria (Lepidoptera: Geometridae).J. Chem. Ecol. 16:1887–1897.
Houx, N. W. H., Voerman, S., andJongen, W. M. F. 1974. Purification and analysis of synthetic insect sex attractants by liquid chromatography on a silver-loaded resin.J. Chromatogr. 96:25–32.
Löfstedt, C. 1991. Evolution of moth pheromones, pp. 57–73,in I. Hrdy (ed.). Insect Chemical Ecology. Academia Praha and SPB Academic Publ., The Hague, The Netherlands.
Löfstedt, C., andKozlov, M. 1996. A phylogenetic analysis of pheromone communication in primitive moths,in R. T. Cardé and A. K. Minks (eds.). Pheromone Research: New Directions. Chapman & Hall, New York (in press).
Löfstedt, C., Elmfors, A., Sjögren, M., andWijk, E. 1986. Confirmation of sex pheromone biosynthesis from (16-D3)-palmitic acid in the turnip moth using capillary gas chromatography.Experientia 42:1059–1061.
Löfstedt, C., Herrebout, W. M., andMenken, S. B. J. 1991. Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae).Chemoecology 2:20–28.
Martinez, T., Fabrias, G., andCamps, F. 1991. Sex pheromone biosynthetic pathway inSpodoptera littoralis and its activation by a neurohormone.J. Biol. Chem. 265:1381–1387.
Minet, J. 1991. Tentative reconstruction of the ditrysian phylogeny (Lepidoptera: Glossata).Entomol. Scand. 22:69–96.
Priesner, E., Naumann, C. M., andStertenbrink, J. 1984. Specificity of synthetic sex attractants inZygaena moths.Z. Naturforsch. 39c:841–844.
Renou, M., Lalanne-Cassou, B., Michelot, D., Gordon, G., andDoré, J. C. 1988. Multivariate analysis of the correlation between Noctuidae subfamilies and the chemical structure of their sex pheromones or male attractants.J. Chem. Ecol. 14:1187–1215.
Roelofs, W. L., andBjostad, L. B. 1984. Biosynthesis of Lepidopteran pheromones.Bioorg. Chem. 12:279–298.
Roelofs, W. L., andBrown, R. L. 1982. Pheromones and evolutionary relationships of Tortricidae.Annu. Rev. Ecol. Syst. 13:395–422.
Rule, G. S., andRoelofs, W. L. 1989. Biosynthesis of sex pheromone components from linolenic acid in the arctiid moths.Arch. Insect Biochem. Physiol. 12:89–94.
Szöcs, G., Töth, M., Bestmann, H. J., Vostrowsky, O., Heath, R. R., andTumlinson, J. H. 1987. Polyenic hydrocarbons as sex attractants for geometrids and amatids Lepidoptera found by field screening in Hungary.Z. Naturforsch. 42c:165–168.
Szöcs, G., Ronkay, L., Vojnits, A., andTöth, M. 1991. Does the chemical structure of sex attractants reflect taxonomical position of geometrid species (Lepidoptera)? pp. 75–80,in I. Hrdy (ed.). Insect Chemical Ecology. Academia Praha and SPB Academic Publ., The Hague, The Netherlands.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, J., Ryrholm, N., Ljungberg, H. et al. Olefinic acetates, Δ-9,11–14: OAc and Δ-7,9–12: OAc used as sex pheromone components in three geometrid moths,Idaea aversata, I. straminata, andI. biselata (Geometridae, Lepidoptera). J Chem Ecol 22, 1505–1526 (1996). https://doi.org/10.1007/BF02027728
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02027728