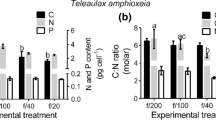
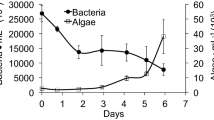
Abstract
The relative importance of autotrophic flagellates, desmids, cyanobacteria, and ciliates as food forDaphnia magna was examined using cohort life tables. Each cohort was fed a single food type at a given concentration, and comparisons among each type were made. Algal feeding treatments included three levels of young (7 to 14 days old)Chlamydomonas reinhardi (Chlorophyta, Chlamydomonadacae), two levels of senescent (> 14 days old)C. reinhardi, two levels ofCryptomonas sp. (Chlorophyta, Cryptomonadacae), two levels ofStaurastrum sp. (Chlorophyta, Desmidacae), four levels of young (7 to 15 days old) or senescent (> 15 days old)Microcystis aeruginosa (Cyanophyta, Chlorococcacae), and a no-food treatment. The ciliatesCyclidium sp. andParamecium caudatum were also presented at concentrations of 1 or 102 cells/ml, as well as mixtures ofC. reinhardi (103/ml) andCyclidium (1/ml) orP. caudatum (1/ml).Daphnia growth, reproduction, and survivorship were highest whenC. reinhardi orCryptomonas were the food source, while those starved or fedM. aeruginosa had shorter survivorship and lower growth and reproduction.Daphnia grew and had high survivorship when fedP. caudatum, but even though eggs were produced, most were aborted after 2 or 3 days.Staurastrum andCyclidium produced intermediate growth and survivorship, but reproduction was seen only in the 103 Staurastrum/ml treatment. Carbon and nitrogen content were general indicators of nutritional value. However, growth, reproduction, and survivorship were higher in some cohorts fed treatments containing relatively low levels of carbon and nitrogen. Other cohorts were short-lived and did not reproduce, despite being fed much higher levels of carbon and nitrogen. The results also suggest that green algae are nutritionally valuable forDaphnia, whereas cyanobacteria are not. As measured by life-table parameters, the nutritional value of ciliates was variable, with some being poor food sources. Thus, the potential of ciliates as a trophic link between microbial production and higher trophic levels may vary with the ciliate community structure. Our results suggest that ciliates alone were insufficient as a food source to supportDaphnia population growth.
Similar content being viewed by others
References
Ambler JW (1986) Effect of food quantity and quality on egg productionAcartia tonsa Dana from East Lagoon, Galveston, Texas. Estuar Coast Shelf Sci 23:183–196
Arnold AE (1971) Ingestion, assimilation, survival, and reproduction byDaphnia pulex fed seven species of blue-green algae. Limnol Oceanogr 16:906–920
Bamforth S (1958) Ecological studies on the planktonic protozoa of a small artificial pond. Limnol Oceanogr 3:398–412
Beaver JR, Crisman TL (1982) The trophic response of ciliated protozoans in freshwater lakes. Limnol Oceanogr 27:246–253
Berk SG, Brownlee DC, Heinle DR, King HJ, Colwell RR (1977) Ciliates as a food source for marine planktonic copepods. Microb Ecol 4:27–40
Blazka P (1966) Metabolism of natural and cultured populations ofDaphnia related to secondary production. Int Ver Theor Angew Limnol Verh 16:380–385
Bratback G, Dundas I (1984) Bacterial dry matter content and biomass estimations. Appl Environ Microbiol 48:755–757
Brooks JL (1946) Cyclomorphosis inDaphnia. I. An analysis ofD. retrocurva andD. galeata. Ecol Monogr 16:409–447
Cahoon LB (1981) Reproductive response ofAcartia tonsa to variations in food ration and quality. Deep-Sea Res 28:1215–1221
Checkley DM Jr. (1980) The egg production of a marine planktonic copepod in relation to its food supply: Laboratory studies. Limnol Oceanogr 25:430–446
DeBernardi R, Giussani G, Lasso Pedretti E (1981) The significance of blue-green algae as food for filter-feeding zooplankton: Experimental studies onDaphnia spp. fed byMicrocystis aeruginosa. Ver Int Ver Theor Angew Limnol 21:477–483
Fulton RS III (1988) Resistance to blue-green algal toxins byBosmina longirostris. J Plank Res 4:771–778
Fulton RS III, Paerl HW (1987) The effects of colonial morphology on zooplankton utilization of algal resources during blue-green algal (Microcystis aeruginosa) blooms. Limnol Oceanogr 32:634–644
Hall DJ (1964) An experimental approach to the dynamics of a natural population ofDaphnia galeata mendotae. Ecology 45:94–112
Haney JW (1987) Field studies on zooplankton-cyanobacteria interactions. New Zeal J Mar Fresh Res 21:467–475
Infante A, Riehl W (1984) The effect of Cyanophyta upon zooplankton in a eutrophic tropical lake (Lake Valencia, Venezuela). Hydrobiologia 113:293–298
Jarvis AC, Hart RC, Combrink S (1987) Zooplankton feeding on size fractionedMicrocystis colonies andChlorella in a hypertrophic lake (Hartbeespoort Dam, South Africa): Implication to resource utilization and zooplankton succession. J Plank Res 9:1231–1249
Lampert W (1977) Studies on the carbon balance ofDaphnia pulex de Geer as related to environmental conditions. 2. The dependence of carbon assimilation on animal size, temperature, food concentration, and diet species. Arch Hydrobiol Suppl 48:310–335
Lampert W (1981) Toxicity of the blue-greenMicrocystis aeruginosa: Effective defense mechanism against grazing pressure byDaphnia. Int Ver Theor Angew Limnol Verh 21:1436–1440
Lampert W (1981) Inhibitory and toxic effects of blue-green algae onDaphnia. Int Rev Gestam Hydrobiol 66:822–830
Lampert W (1982) Further studies on the inhibitory effect of the toxic blue-greenMicrocystis aeruginosa on the filtering rate of zooplankton. Arch Hydrobiol 95:207–220
Lampert W (1987) Laboratory studies on zooplankton-cyanobacteria interactions. New Zeal J Mar Fresh Res 21:483–490
Mattson WJ Jr. (1980) Herbivory in relation to plant nitrogen content. Ann Rev Ecol Syst 11:119–161
Nizan S, Dimentman C, Shilo M (1986) Acute toxic effects of the cyanobacteriumMicrocystis aeruginosa onDaphnia magna. Limnol Oceanogr 31:497–502
Pace ML (1982) Planktonic ciliates: Their distribution, abundance, and relationship to microbial resources in a monomictic lake. Can J Fish Aquat Sci 39:1106–1116
Pace ML, Orcutt JD (1981) The relative importance of protozoans, rotifers, and crustaceans in a planktonic community. Limnol Oceanogr 26:822–830
Porter KG (1975) Viable gut passage of gelatinous green algae ingested byDaphnia. Int Theor Angew Limnol Verh 19:2840–2850
Porter KG, Pace ML, Battey JF (1979) Ciliate protozoans as links in freshwater planktonic food chains. Nature 277:563–565
Porter KG, Orcutt JD (1980) Nutritional adequacy, manageability, and toxicity as factors that determine the food quality of green and blue-green algae forDaphnia. In: Kerfoot WC (ed) Evolution and ecology of zooplankton communities. University Press of New England, Hanover, New Hampshire, pp 268–281
Porter KG, Orcutt JD, Gerritsen J (1983) Functional response and fitness in a generalist filter feeder,Daphnia magna (Cladocera:Crustacea). Ecology 64:735–742
Porter KG, McDonough RJ (1984) The energetic cost of response to blue-green algal filaments by cladocerans. Limnol Oceanogr 29:365–369
Porter KG, Sherr EB, Sherr BF, Pace ML, Sanders RW (1985) Protozoa in planktonic food webs. J Protozool 32:409–415
Redfield GW (1981) Nutrition and the degeneration of eggs in a limnetic daphnid. Ver Internat Verein Limnol 21:1550–1554
Sanders RW, Porter KG, Bennett SJ, DeBiase AE (1989) Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnol Oceanogr 34:673–687
Sanders RW, Porter KG (1990) Bacterivorous flagellates as food resources for the freshwater zooplankterDaphnia ambigua. Limnol Oceanogr (in press)
Schindler DW (1968) Feeding, assimilation, and respiration rates ofDaphnia magna under various environmental conditions and their relation to production estimates. J Anim Ecol 37:369–385
Sherr EB, Sherr BF, Paffenhöffer GA (1986) Phagotrophic protozoa as food for metazoans: A “missing” trophic link in marine pelagic food webs? Mar Microb Food Webs 1:61–80
Smith GH (1950) The fresh-water algae of the United States. McGraw-Hill Co., New York
Starkweather PL (1981) Trophic relationship between the rotiferBrachionus calyciflorus and the blue-green algaAnabaena flos-aquae. Ver Int Verein Limnol 21:1507–1514
Starkweather PL, Kellar PE (1983) Utilization of cyanobacteria byBrachionus calyciflorus: Anabaena flos-aquae (NRC-44-1) as a sole or complementary food source. Hydrobiologia 104:373–377
Strangenberg M (1968) Toxic effects ofMicrocystis aeruginosa Kg. extracts onDaphnia longispina O. F. Muller andEucypris virens Jurine. Hydrobiologia 32:81–87
Tezuka Y (1971) Feeding ofDaphnia on planktonic bacteria. Jpn J Ecol 21:127–134
Tezuka Y (1974) An experimental study on the food chain among bacteria,Paramecium, andDaphnia. Int Rev Gestam Hydrobiol 59:31–37
Threlkeld ST (1979) Estimating cladoceran birth rates: The importance of egg mortality and the egg age distribution. Limnol Oceanogr 21:601–612
Vijverberg J (1976) The effect of food quantity and quality on the growth, birth-rate and longevity ofDaphnia hyalina Leydig. Hydrobiologia 51:99–108
Webster KE, Peters RH (1978) Some size-dependent inhibitions of larger cladoceran filterers in filamentous suspensions. Limnol Oceanogr 23:1238–1245
Wetzel R (1983) Limnology. WB Saunders Co., Philadelphia, Pennsylvania
Wilbert N (1969) Okologische Untersuchung der Aufwuchs- und Plankton-Ciliaten eines Eutrophen Weihers. Arch Hydrobiol Suppl 35:411–518
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DeBiase, A.E., Sanders, R.W. & Porter, K.G. Relative nutritional value of ciliate protozoa and algae as food forDaphnia . Microb Ecol 19, 199–210 (1990). https://doi.org/10.1007/BF02012100
Issue Date:
DOI: https://doi.org/10.1007/BF02012100