Abstract
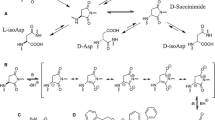
To study the mechanism of protein carboxyl methyltransferase-driven repair of age-damaged sites in polypeptides, a modell-isoaspartyl peptide,l-isotetragastrin, was enzymatically repaired to normall-tetragastrin in the presence of18O-enriched water. By this design, the enrichment of18O atoms in the peptide would reflect the number of passages through a hydrolyzable succinimide intermediate during formation of the repaired product. Mass determinations by FAB mass spectrometry revealed repaired peptide with two18O atoms incorporated, demonstrating that more than a single cycle of methylation and demethylation is necessary to ensure stoichiometric repair.
Similar content being viewed by others
Abbreviations
- HPLC:
-
high-pressure liquid chromatography
- FAB:
-
fast atom bombardment
- TFA:
-
trifluoroacetic acid
- PCM:
-
proteind-aspartyl/L-isoaspartyl carboxyl methyltransfer-ase
- l-Normal:
-
[l-Asp3]tetragastrin
- l-Iso:
-
[L-isoAsp3]tetragastrin
- d-Normal:
-
[d-Asp3]tetragastrin
- d-Iso:
-
[d-isoAsp3]tetragastrin
References
Aswad, D. W. (1984).J. Biol. Chem. 259, 10714–10721.
Atkinson, D. E. (1977).Cellular Energy Metabolism and Its Regulation, Academic Press, New York.
Gilbert, J. M., Fowler, A., Bleibaum, J., and Clarke, S. (1988).Biochemistry 27, 5227–5233.
Gonzalez, J., Takao, T., Hori, H., Besada, V., Rodriguez, R., Padron, G., and Shimonishi, Y. (1992).Anal. Biochem. 205, 151–158.
Ingrosso, D., Kagan, R. M., and Clarke, S. (1991).Biochem. Biophys. Res. Commun. 175, 351–358.
Johnson, B. A., and Aswad, D. W. (1990). InProtein Methylation (Paik, W. K., and Kim, S., eds.), CRC Press, Boca Raton, Florida, pp. 195–210.
Johnson, B. A., Langmack, E. L., and Aswad, D. W. (1987a).J. Biol. Chem. 262, 12283–12287.
Johnson, B. A., Murray, E. J., Clarke, S., Glass, D. B., and Aswad, D. W. (1987b).J. Biol. Chem. 262, 12283–12287.
Johnson, B. A., Murray, E. J., Clarke, S., Glass, D. B., and Aswad, D. W. (1987b).J. Biol. Chem. 262, 5622–5629.
Kaartinen, V., Mononen, T., Laatikainen, R., and Mononen, I. (1992).J. Biol. Chem. 267, 6855–6858.
Lindquist, J. A. and McFadden, P. N. (1994).J. Protein Chem. 13, 23–30.
MacLaren, D. C., Kagan, R. M., and Clarke, S. (1992).Biochem. Biophys. Res. Commun. 185, 277–283.
McFadden, P. N., and Clarke, S. (1982).Proc. Natl. Acad. Sci. USA 79, 2460–2464.
McFadden, P. N., and Clarke, S. (1986).J. Biol. Chem. 261, 11503–11511.
McFadden, P. N., and Clarke, S. (1987).Proc. Natl. Acad. Sci. USA 84, 2595–2599.
Murray, E. J., and Clarke, S. (1984).J. Biol. Chem. 259, 10722–10732.
Ota, I. M., and Clarke, S. (1990). InProtein Methylation (Paik, W. K., and Kim, S., eds.), CRC Press, Boca Raton, Florida, pp. 179–194.
Rose, K., Savoy, L. A., Simona, M. G., Offord, R. E., and Wingfield, P. (1988).Biochem. J. 250, 253–259.
Visentini, J., Paul, G. J. C., and Bertrand, M. J. Proceedings of the 39th ASMS Conference on Mass Spectrometry and Allied Topics, Nashville, TN (1991). 1075–1076.
Wright, H. T. (1991).Crit. Rev. Biochem. Mol. Biol. 26, 1–52.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindquist, J.A., McFadden, P.N. Incorporation of two18O atoms into a peptide during isoaspartyl repair reveals repeated passage through a succinimide intermediate. J Protein Chem 13, 553–560 (1994). https://doi.org/10.1007/BF01901537
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01901537