Summary
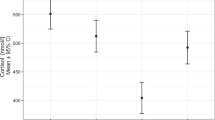
We determined the adrenostatic potential of low-dose nonhypnotic etomidate in six patients with Cushing's syndrome (ectopic Cushing's syndrome,n=2; Cushing's disease,n=3; bilateral adrenal adenoma,n=1). Etomidate was given as a continuous infusion for 32 h in a dose of 2.5 mg/h (n=5) or 0.3 mg/kg/h (n=3), respectively. Saline was given during a control period. The responsiveness to exogenous ACTH was studied during placebo and 7 and 31 h after commencing etomidate by administration of 250 µg 1–24 ACTH i.v. Etomidate (2.5 mg/h) led to a consistent decrease in serum cortisol in all patients from a mean of 39.4±13.3 to 21.1±5.7 µg/dl after 7 h (P<0.05 compared with placebo). After 24 h cortisol was reduced further to a mean steady state concentration of 12.3±5.7 µg/dl (P<0.05). At the end of the infusion period the cortisol increase in response to ACTH was reduced but not abolished. In contrast, a dose of 0.3 mg/kg/h etomidate induced unresponsiveness of serum cortisol to exogenous ACTH within 7 h. However, sedation was observed in two out of three patients at this dose, while during etomidate in a dose of 2.5 mg/h no side effects were seen. We conclude that low-dose non-hypnotic etomidate reduces serum cortisol to within the normal range in patients with Cushing's syndrome. The possibility to dissociate the adrenostatic effect of etomidate from its hypnotic action, the absence of side effects, and the i.v. route suggest that etomidate in a dose of 0.04–0.05 mg/kg/h may become the drug of choice for rapid initial control of hypercortisolism.
Similar content being viewed by others
Abbreviations
- ACTH:
-
adrenocorticotrophic hormone
- CD:
-
Cushing's disease
- CS:
-
Cushing's syndrome
References
Allolio B, Winkelmann W, Hipp FX (1980) Effect of meclastine, an H1-antihistamine, on plasma ACTH in adrenal insufficiency. Acta Endocrinol 96:98–101
Allolio B, Stuttmann R, Leonhard U, Fischer H, Winkelmann W (1984) Adrenocortical suppression by a single induction dose of etomidate. Klin Wochenschr 62:1014–1017
Allolio B, Dörr H, Stuttmann R, Knorr D, Engelhardt D, Winkelmann W (1985) Effect of a single bolus of etomidate upon eight major corticosteroid hormones and plasma ACTH. Clin Endocrinol 22:281–286
Child DF, Burke CW, Burley DW, Rees LH, Fraser TR (1985) Drug control of Cushing's syndrome. Acta Endocrinol 82:330–341
De Coster R, Beerens D, Haelterman C, Wouters L (1985) Effects of etomidate on cortisol biosynthesis in isolated guinea-pig adrenal cells: comparison with metyrapone. J Endocrinol Invest 8:199–202
Engelhardt D, Stuttmann H, Jacob K, Dörr HG (1986) Influence of low-dose etomidate on adrenal and gonadal steroid hormone secretion in normal men. Acta Endocrinol [Suppl 274] 111:21–22
Fragen RJ, Shanks CA, Molteni A, Avram MJ (1984) Effects of etomidate on hormonal responses to surgical stress. Anesthesiology 61:652–656
Fry DE, Griffiths H (1984) The inhibition by etomidate of the 11β-hydroxylation of cortisol. Clin Endocrinol 20:625–629
Gärtner R, Albrecht M, Müller OA (1986) Effect of etomidate on hypercortisolism due to ectopic ACTH production. Lancet I:275
Hebron BS, Edbrooke DL, Newby DM, Mather SJ (1983) Pharmacokinetics of etomidate associated with prolonged i.v. infusion. Br J Anaesth 55:281–285
Jeffcoate WJ, Rees LH, Tomlin S, Jones AE, Edwards CRW, Besser GM (1977) Metyrapone in long-term management of Cushing's disease. Br Med J 2:215–217
Kenyon CJ, Young J, Gray CE, Fraser R (1984) Inhibition by etomidate of steroidogenesis in isolated bovine adrenal cells. J Clin Endocrinol Metab 58:947–949
Lambert A, Mitchell R, Forst J, Ratcliffe JG, Robertson WR (1983) Direct in vitro inhibition of adrenal steroidogenesis by etomidate. Lancet II:1085–1086
Loli P, Berselli ME, Tagliaferri M (1986) Use of ketoconazole in the treatment of Cushing's syndrome. J Clin Endocrinol Metab 63:1365–1370
Stalla GK, Stalla J, Huber M, Müller OA (1987) Ketoconazole inhibits cAMP generation and ACTH secretion in rat anterior pituitary cell culture. Acta Endocrinol [Suppl 283] 114:32–33
Thoren M, Adamson U, Sjöberg HE (1985) Aminoglutethimide and metyrapone in the management of Cushing's syndrome. Acta Endocrinol 109:451–457
Vanden Bossche H, Willemsens G, Cools W, Bellens D (1984) Effects of etomidate on steroid biosynthesis in subcellular fractions of bovine adrenals. Biochem Pharmacol 33:3861–3868
Voigt KH, Fehm HL, Reck R, Pfeiffer EF (1974) Spontaneous and stimulated secretion of Quso-extractable immunoassayable ACTH in man. Klin Wochenschr 52:516–521
Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman MD (1984) Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med 310:1415–1421
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allolio, B., Schulte, H.M., Kaulen, D. et al. Nonhypnotic low-dose etomidate for rapid correction of hypercortisolaemia in cushing's syndrome. Klin Wochenschr 66, 361–364 (1988). https://doi.org/10.1007/BF01735795
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01735795