Abstract
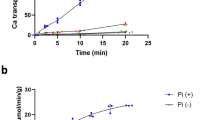
Escherichia coli grown anaerobically for osmotic studies upon increased osmolarity in alkaline medium carried out H+−K+-exchange in two steps, the first of which was DCCD1 sensitive and osmo-dependent and had the 2H+/K+ stoichiometry. H+-efflux in the presence of protonophore (CCCP) upon increase of osmolarity was shown to be high and inhibited by DCCD, whereas H+-efflux induced by a decrease of osmolarity was small and not inhibited by DCCD. The 2H+/K+-exchange was absent intrkA anduncA mutants. InuncB mutant 2H+/K+-exchange was not DCCD-and osmosensitive. Competition between DCCD and osmoshock on inhibition of 2H+/K+-exchange was found. Osmosensitivity of this exchange disappeared in spheroplasts. Osmosensitivity of both 2H+/K+-exchange and the F0F1 and osmoregulation of the F0F1 via F0 and a periplasmic space are postulated.
Similar content being viewed by others
Abbreviations
- F0F1 :
-
H+-ATPase complex
- F0 :
-
H+-channel, proteolipid
- F1 :
-
H+-ATPase
- Trk :
-
constitutive system for K+ uptake
- PV:
-
periplasmic protein valve
- DCCD:
-
N,N′-dicyclohexylcarbodiimide
- CCCP:
-
carbonylcyanide-m-chlorophenylhydrazone
- ΔμH or ΔμK :
-
transmembrane electrochemical gradient for H+ or K+ respectively
- ΔΦ:
-
membrane potential
- upshock or downshock:
-
increase or decrease of medium osmolarity, respectively
- CGSC:
-
E. coli Genetic Stock Center, Yale University, USA
Literature Cited
Bagramyan KA, Martirosov SM (1989) Formation of an ion transport supercomplex inEscherichia coli. an experimental model of direct transduction of energy. FEBS Lett 246:149–152
Bakker EP (1981) The role of ATP and the proton-motive force in potassium transport by theEscherichia coli TrkA system. In: Palmieri F (ed) Vectorial reactions in electron and ion transport in mitochondria and bacteria. Amsterdam; Holland Biomed Press, pp 315–321
Dosch DC, Helmer GL, Sutton RH, Salvacion FF, Epstein W (1991) Genetic analysis of potassium transport loci inEscherichia coli. Evidence for three constitutive systems mediating uptake of potassium. J Bacteriol 173:687–696
Dunn S (1978) Identification of the altered subunit in the inactive F1-ATPase of anEscherichia coli uncA mutant. Biochem Biophys Res Commun 82:596–602
Durgaryan SS, Martirosov SM (1978a) An electrochemical study of energy-dependent potassium accumulation inE. coli. 1. Two steps in potassium uptake and osmosensitivity. Bioelectrochem Bioenerg 5:554–560
Durgaryan SS, Martirosov SM (1978b) An electrochemical study of energy-dependent potassium accumulation inE. coli. 3. Stoichiometry of H+−K+-exchange sensitive to N,N′-dicyclohexylcarbodiimide. Bioelectrochem Bioenerg 5:567–573
Epstein W (1986) Osmoregulation by potassium transport inEscherichia coli. FEMS Microbiol Rev 39:73–78
Epstein W (1990) Bacterial transport ATPases. In: Krulwich TA (ed) Bacterial energetics. The bacteria, vol. 12. San Diego: Academic Press, pp 87–110
Gibson F (1983) Biochemical and genetic studies on the assembly and function of the F1F0 adenosine triphosphatase ofEscherichia coli. Biochem Soc Trans 11:229–240
Gibson F, Cox GB, Downie, JA, Radick J (1978) Partial diploids ofEscherichia coli carrying normal and mutant alleles affecting oxidative phosphorylation. Biochem J 162:665–672
Kaback HR (1971) Bacterial membranes. Methods Enzymol 22:99–120
Martirosov SM (1979) An electrochemical studys of energydependent potassium accumulation inE. coli. 4. Regulation of the H+−K+-pump operation by a periplasmic protein. Bioelectrochem Bioenerg 6:315–321
Martirosov SM, Trchounian AA (1981a) An electrochemical study of energy-dependent potassium accumulation inE. coli. 5. Electrogenic proton pump and lactate secretion. Bioelectrochem Bioenerg 8:25–32
Martirosov SM, Trchounian AA (1981b) An electrochemical study of energy-dependent potassium accumulation inE. coli. 6. Identification of H+−K+-exchanging systems. Bioelectrochem Bioenerg 8:597–603
Martirosov SM, Trchounian AA (1981c) An electrochemical study of energy-dependent potassim accumulation inE. coli. 7. On the structure of H+−K+-exchanging systems. Bioelectrochem Bioenerg 8:605–611
Martirosov SM, Trchounian AA (1982) An electrochemical study of energy-dependent potassium accumulation inE. coli. 9. Reversal of the mechanism exchanging 2H+ for K+ with the coupled synthesis of ATP. Bioelectrochem Bioenerg 9:459–467
Martirosov SM, Trchounian AA (1983) An electrochemical study of energy-dependent potassium accumulation inE. coli. 10. Operation of transport systems exchanging H+ for K+ inunc-mutants. Bioelectrochem Bioenerg 11: 29–36
Martirosov SM, Trchounian AA (1986) An electrochemical study of energy-dependent potassium accumulation inE. coli. 11. TheTrk system in anaerobically and aerobically grown cells. Bioelectrochem Bioenerg 15:417–426
Martirosov SM, Petrosian LS, Trchounian AA, Vardanian AG (1981) An electrochemical study of energy-dependent potassium accumulation inE. coli. 8. Membrane potential (comparison withS. faecalis). Bioelectrochem Bioenerg 8:613–620
Martirosov SM, Ogandjanian ES, Trchounian AA (1988) An electrochemical study of energy-dependent potassium accumulation inE. coli. 12. K+-dependent ATPase activity. Bioelectrochem Bioenerg 19:353–357
Rhoads DB, Epstein W (1977) Energy coupling to net K+ transport inEscherichia coli K12. J Biol Chem 252:1394–1401
Rhoads DB, Epstein W (1978) Cation transport inEscherichia coli. 9. Regulation of K+ transport. J Gen Physiol 72:283–295
Rhoads DB, Waters FB, Epstein W (1976) Cation transport inEscherichia coli. 8. Potassium transport mutants. J Gen Physiol 67:325–341
Sebald W, Hoppe J (1981) On the structure and genetics of the proteolipid subunits of the ATP synthase complex. Curr Top Bioenerg 12:1–64
Trchounian AA, Ogandjanian ES (1989) On the reason of the principle of intramembranal interaction of transport systems in bacteria. Stud Biophys 132:231–234
Trchounian AA, Vassilian AV (1992) Potassium-stimulated N,N'-dicyclohexylcarbodiimide-sensitive ATPase activity in anaerobically grownEscherichia coli. Ann NY Acad Sci 671:490–492
Trchounian AA, Ogandjanian ES, Mironova GD (1992) An electrochemical study of energy-dependent potassium accumulation inE. coli. 13. On the interaction of the H+-ATPase complex F0F1 with theTrk proteins in anaerobically grown cells. Bioelectrochem Bioenerg 27:267–272
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trchounian, A.A., Ogandjanian, E.S. & Vanian, P.A. Osmosensitivity of the 2H+−K+-exchange and the H+-ATPase complex F0F1 in anaerobically grownEscherichia coli . Current Microbiology 29, 187–191 (1994). https://doi.org/10.1007/BF01570152
Issue Date:
DOI: https://doi.org/10.1007/BF01570152