Abstract
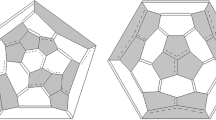
Semi-empirical (MNDO) and ab-initio Hartree-Fock (3-21G) calculations on the structure and stability of C72 are reported. Though never isolated experimentally, C72 has been previously suggested to be a particularly stable fullerene based on simple Hückel molecular orbital ideas. However, our calculations which include complete geometry optimization definitively show that it is significantly less stable than C70. The structural and electronic factors which are responsible for its instability are discussed.
Similar content being viewed by others
References
W. Krätschmer, L. D. Lamb, K. Fostiropoulos, and D. R. Huffman, Nature 347 (1990) 354.
R. Taylor, J. P. Hare, A. K. Abdul-Sada, and H. W. Kroto, J. Chem. Soc. Chem. Commun. (1990) 1423.
F. Diederich, R. L. Whetten, C. Thilgen, R. Ettl, I. Chao, and M. M. Alvarez, Science, 254 (1991) 1768.
F. Diederich, R. Ettl, Y. Rubin, R. L. Whetten, R. Beck, M. Alvarez, S. Anz, D. Sensharma, F. Wudl, K. C. Khemani, and A. Koch, Science 252 (1991) 548
R. Ettl, I. Chao, F. Diederich, and R. L. Whetten, Nature, 353 (1991) 149.
K. Kikuchi, N. Nakahara, T. Wakabayashi, S. Suzuki, H. Shiromaru, Y. Miyake, K. Saito, I. Ikemoto, M. Kainosho, and Y. Achiba, Nature, 357 (1992) 142.
S. Hino, K. Matsumoto, S. Hasegawa, K. Kamiya, H. Inokuchi, T. Morikawa, T. Takahashi, K. Seki, K. Kikuchi, S. Suzuki, I. Ikemoto, and Y. Achiba, Chem. Phys. Lett. 190 (1992) 169.
H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl, and R. E. Smalley, Nature 318, (1985) 162.
H. W. Kroto, Nature 329, (1987) 529.
T. G. Schmalz, W. A. Seitz, D. J. Klein, and G. E. Hite, J. Am. Chem. Soc. 110, (1988) 1113.
P. W. Fowler, J. Chem. Soc. Faraday Trans. 87 (1991) 1945.
P. W. Fowler, D. E. Manolopoulos, and R. C. Batten, J. Chem. Soc. Faraday Trans. 87 (1991) 3103.
D. E. Manolopoulos, J. Chem. Soc. Faraday Trans. 87 (1991) 2861.
R. Taylor, J. Chem. Soc. Perkin Trans. (1992) 3.
X. Liu, T. G. Schmalz, and D. J. Klein, Chem. Phys. Lett. 188 (1992) 550.
P. W. Fowler and D. E. Manolopoulos, Nature, 355 (1992) 428.
K. Raghavachari and C. M. Rohlfing, J. Phys. Chem. 95 (1991) 5768.
K. Raghavachari and C. M. Rohlfing, J. Phys. Chem. 96 (1992) 2463.
K. Raghavachari, Mat. Res. Soc. Symp. Proc. (1992, in press).
K. Raghavachari, Chem. Phys. Lett. 190 (1992) 397.
X.-Q. Wang, C. Z. Wang, B. L. Zhang, and K. M. Ho, Phys. Rev. Lett. 69 (1992) 69.
B. L. Zhang, C. Z. Wang, and K. M. Ho, Chem. Phys. Lett. 193 (1992) 225.
M. J. S. Dewar and W. Thiel, J. Am. Chem. Soc. 99 (1977) 4899.
W. J. Hehre, L. Radom, P. v. R. Schleyer, and J. A. Pople,Ab Initio Molecular Orbital Theory (John Wiley, New York, 1986).
All calculations performed using Gaussian 90 Computer Program, M. J. Frisch, M. Head-Gordon, G. W. Trucks, J. B. Foresman, H. B. Schlegel, K. Raghavachari, M. Robb, J. S. Binkley, C. Gonzalez, D. J. Defrees, D. J. Fox, R. A. Whiteside, R. Seeger, C. F. Melius, J. Baker, R. L. Martin, L. R. Kahn, J. J. P. Stewart, S. Topiol, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1990.
R. C. Haddon and L. T. Scott, Pure Appl. Chem. 58 (1986) 137.
K. Raghavachari and C. M. Rohlfing, (to be published).